|
Introduction | Back Ground | The Study | Results | Evidence | Discussion and Conclusion | My Comments
Hirsch JA, Singh V, Manchikanti L, et al. “Automated Percutaneous Lumbar Discectomy For the Contained Herniated Lumbar Disc A Systematic Assessment of Evidence." Pain Physician 2009; 12:601-620.
INTRODUCTION:
In 2009, Hirsch et al. published the results of their systematic review into the evidence-based efficacy of automated percutaneous lumbar discectomy (APLD) as a treatment intervention for contained disc herniation. Only the most scientific, evidence-based research papers were allowed entry into this "study of studies," which makes its conclusions quite powerful.
APLD, which is classified as a "minimally invasive procedure," is particularly interesting, for it has been performed on more patients worldwide than any other of its class [60]. More specifically, there were 40,000 patients treated with APLD by 1990 [126].
What is APLD?
But why do we need APLD when we already have a gold standard treatment for disc herniation, i.e., the open discectomy or microdiscectomy, which are both equally effective [1]? We need this class of procedures because open discectomy/microdiscectomy do not work for all types of herniation.
Digression: More specifically, a certain flavor (class) of disc herniation called "small contained herniation" response very poorly to traditional open discectomy/microdiscectomy [10, 11, 12, 13]. Famed award-winning researcher and author Eugene Carragee discovered that not all small contained herniations, however, responded poorly to open discectomy/microdiscectomy. In his 2003 paper [13], he discovered two classes of small contained herniation: the "fragment contained-herniation," and the "no fragment contained-herniation." [see my review of that paper here] and only the latter responded poorly to open discectomies/microdiscectomy. no fragment contained-herniations look something like this:
 |
This drawing depicts Carragee's no fragment contained-herniation, which responds very poorly to microdiscectomy.
Notice that the annular fissure is very small (#8) and not extending into the outer third of the annulus fibrosis, which is where all the pain-carrying nerve fiber lives.
It is believed that this type of herniation, which, unfortunately, can only be detected under discography, would respond very well to pressure-reducing, minimally invasive treatment intervention, such as Automated Percutaneous Lumbar Discectomy (APLD). |
BACKGROUND AND HISTORY OF PERCUTANEOUS LUMBAR DISCECTOMY:
In the 1970s, Kambin and Gellmann (United States) [301] and Hijikata (Japan) [302] independently performed a non-visualized, manual percutaneous nucleotomy via a posterolateral approach. These early techniques were best described as a manual percutaneous disc decompression which used instrumentation such as an annulus cutter, forceps and graspers to ultimately cut and remove nucleus pulposus from the center of the disc. One downfall to the procedure was the repeated in-and-out movement the probe through the disc, which obviously is not good for the annulus.
In that publication [302], Hijikata explained that this blind technique resected the nuclear substance of the disc—not the herniated portion—in order to reduced intradiscal pressure, which in turn retracted the contained herniation back into the disc and relieved compressive forces on pain-carrying nerve receptors in the periphery of the disc. Initial results of the procedure were good, for of the 136 cases followed in that review, 72% of the patients were satisfied with the outcome of the procedure [302].
Variations of this manual technique were subsequently introduced [57, 58]. Unfortunately, serious complications were reported these early procedures, such as vascular injuries and discitis, which sparked the need for further innovation.
 In 1984, Onik et al [304, 305, 314] published a paper describing an automated percutaneous lumbar discectomy (APLD), which used a 2.5 mm cannula (much smaller than the old cannulas that were greater than 5mm in diameter) to deliver a 2mm patented device called the "Nucleotome aspiration probe (Clarus Medical)." Onik, who recognize the similarity between vitreous material of the eye and that of the disc nucleus, worked with engineers to modify ophthalmic equipment into a device that could be used through a cannula, which ultimately became known as Nucleotome (63-65). In 1984, Onik et al [304, 305, 314] published a paper describing an automated percutaneous lumbar discectomy (APLD), which used a 2.5 mm cannula (much smaller than the old cannulas that were greater than 5mm in diameter) to deliver a 2mm patented device called the "Nucleotome aspiration probe (Clarus Medical)." Onik, who recognize the similarity between vitreous material of the eye and that of the disc nucleus, worked with engineers to modify ophthalmic equipment into a device that could be used through a cannula, which ultimately became known as Nucleotome (63-65).
The Nucleotome probe functions by aspirating nucleus pulposus material through a side port by a suck-and-cut methodology—i.e., nucleus pulposus tissue is sucked inside the probe and then chopped up by a guillotine-like inner cutting sleeve. The destroyed disc material is then carried away through the probe in a saline solution. The beauty of this system is that the nucleus pulposus (center of the disc tissue) is loose enough to be pulled into the probe; whereas, the collagenous annulus fibrosis is not, and in turn spared from the cutting action.
Yet another plus for the procedure over the earlier manual procedures, is the fact that once the probe enters the central portion of the disc, it remains there until all of the sucking-and-cutting (aspiration) has been completed. The probe does not have to be removed and replaced multiple times like in the older versions of this procedure, which will diminish the chance of discitis, vascular injury, nerve injury, as well as mitigate structural damage to the annulus [156].
Currently, this methodology that was first introduced by Onik et al. was the most widely used method of percutaneous discectomy [60]; however, it lost popularity after a randomized controlled trial was published in 1993 [66], which compared chemo nuclear is with APLD. In this study, only a 33% success rate was demonstrated for nucleoplasty. In 1995, Chatterjee et al [67] compared APLD with discectomy. Their results showed only a 29% success rate with nucleoplasty--this further cooled the enthusiasm for this procedure.
The Best Candidates:
The best candidates for this procedure, as noted above, are those with small contained disc herniations that harbors an incomplete annular tear as demonstrated on discography; the disc should have a minimal amount of degeneration and should not be decreased in height very much. With regard to symptomatology, it is even better to have symptoms of leg pain greater than low back pain [60, 61]. This procedure is likely to fail in patients who have disc extrusions (aka, non-contained herniations) or sequestrations [62].
Why does pressure build within the disc with the passage of time? Onik's explanation [304] was that the intervertebral disc may be considered an osmotic system because of its high water content. And everything is happily in balance until the macromolecules of the disc began to break down (secondary to aging and degenerative change) during the fourth and fifth decades of life. This breakdown of molecules increases the number of disc particles that, secondary to osmosis, draws water into the disc raising intradiscal pressure. This increase in intradiscal pressure increases the chance of the development of a pressure reducing annular tear, which unfortunately often become symptomatic and leads to disc herniation.
APLD: How Does It Work?
In laymen's terms, this outpatient procedure, which is done under local anesthesia, starts when the physician places a guide-needle into the center of the disc (i.e., the nucleus pulposus). Once it is confirmed to be in place by fluoroscopy, a 2.8 mm cannula is inserted and the needle is withdrawn. Then a pneumatically driven, suction-cutting probe (one such devices called the Nucleotome) is placed through the cannula into the center of the disc where it is activated by the physician. When activated it sucks nuclear material (which is soft and pliable compared to the tough annulus) into the cannula where it is cut loose and sucked out through the cannula through the probe. All in all, approximately 3 g of disc material is removed in total [17-19, 126], which is enough to affect a diminishment in intradiscal pressure. And it is this decrease in the intradiscal pressure that ultimately has the curing effect to relieve patients of there back and leg pain. It is important to understand that this procedure does not take out the herniated nuclear material (like a traditional microdiscectomy would); instead, it removes the center of the disc (nucleotomy) in order to decrease the volume of the disc. This decrease in volume greatly reduces intradiscal pressure, which in turn will retract (suck back in) the herniated portion of the disc thereby reducing nerve root compression. The volume decrease will also relieve pressure on the pain-sensitive sinuvertebral nerve receptors, which are located in the periphery of the annulus fibrosis.
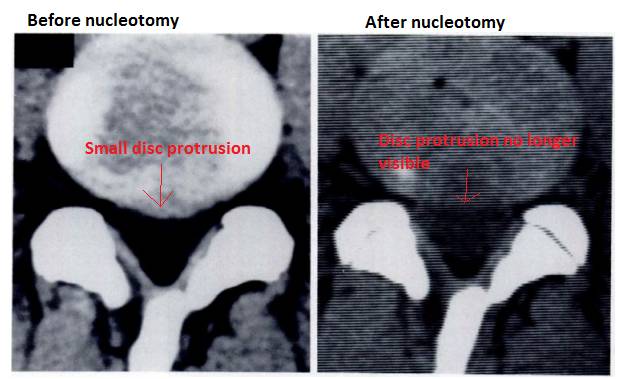 |
Here we have a broad based disc protrusion (left), which was reduced (right) (retracted/sucked back into the disc's center) following automated percutaneous lumbar discectomy (APLD). |
THE STUDY:
This research team performed a thorough search of the medical literature via PubMed, EMBASE, The Cochrane Library, and the Database of Reviews of Effectiveness (DARE) for all research papers concerning APLD. The initial collection of material was cut down by applying the standard "Methodological Quality Assessment" described by the Agency of Healthcare Research and Quality (AHRQ) [65] for observational studies (see below further assessment tool).
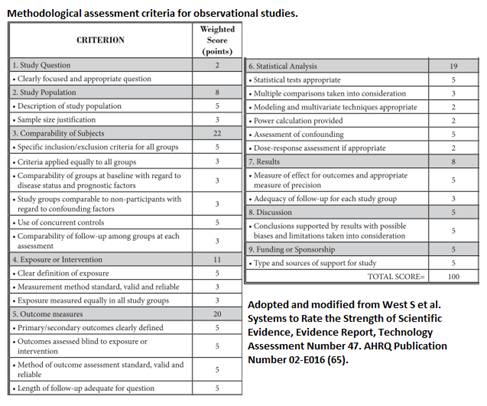
For the available randomized control trials, methodological quality assessment was performed by utilizing the Cochrane review criteria [64]. (See below further assessment tool).
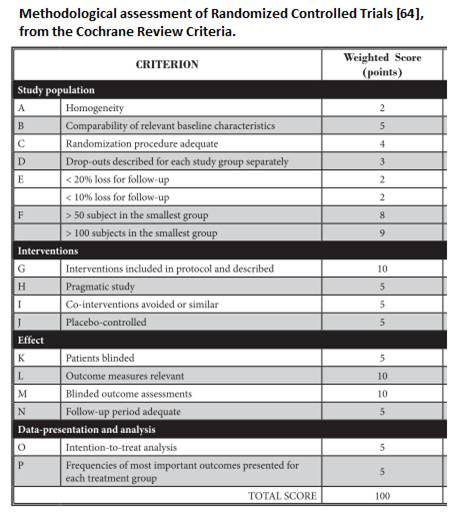
In order to qualify for entry into this systematic review, research papers had to score at least 50/100 points.
In order to assess the final efficacy of APLD, pain relief , typically report by the Visual Analog Scale (VAS) was the primary outcome measure. Secondary outcome measures included functional improvement, improvement of psychological status, and return to work.
With regard to the primary outcome tool, VAS improvement, a higher standard of "success" was utilized in this study in accord with opinions from multiple authors [86-95].
RESULTS:
Of the 80 articles discovered in the research search, which included four randomized controlled trials, two randomized control trials and 12 observational studies were of high enough scientific quality to make it into this systematic review.
The first randomized controlled trial [53] randomly assigned patients with disc-herniation-induced sciatica into a chemonucleolysis group (n=72) or an APLD group (n=69). For some strange reason, the chemonucleolysis group received a statistically higher proportion of patients of whom had more severe degenerative changes (one of the contraindications for APLD). Another problem with this study was the fact that 32 (23%) patients had to withdraw the trial because of therapeutic failure. Notwithstanding those technical problems, the authors of the study concluded that both treatment interventions performed disappointingly. More specifically, 48% of the overall population entering the study considered the treatment of failure and 20% were lost to open discectomy within six months. And to make things even worse, at the one year time point, the overall success rate of chemonucleolysis was 66%, versus only 37% in the APLD group. Many authors attributed this poor success rate to poor patient selection (the included patients with herniations bigger than 30% of the spinal canal, as well as herniations that had migrated caudally or cranially, which many consider a contraindication for APLD. Other negative factors included the fact they allowed patients in who had grade 5 annular tears (i.e. there was epidural leakage of contrast material upon disc injection). They also allowed patients in who had marked disc space narrowing, severe low back pain with no corresponding leg pain. So this is an example of what can go wrong if patient selection is not strictly monitored.
However, Chatterjee et al. [51] compared APLD to microdiscectomy for the treatment of contained disc herniations. This was a randomized study with the blind assessment. This study used very strict criteria for entry: the patient had to have radicular complaints greater than axial low back pain. They had to fail at least six weeks of conservative care. The disc herniations had to be less than 30% the canal size and only at a single level. Bulging discs were allowed in. Notwithstanding these strict entry criteria, only 29% of the patients reported a satisfactory outcome after APLD, compared to 80% in the microdiscectomy group. These researchers concluded APLD was an ineffective method for the treatment of small contained disc herniations. The group was criticized, however for they failed to utilize CT discography.
The observational studies, however, fared much better in supporting APLD as an efficacious treatment intervention for small contained disc herniation. There were a large number of studies that attested to the success of APLD. More specifically, they showed an approximate overall success rate of 75% with relatively low risk factors.
One of the best studies was from the pioneer of APLD, Onik et al [22]. He carried out a multi-institutional study on 327 patients. The procedures were performed by 18 different surgeons within this prospective observational study. At the one year time point, the success rate was 75.2%. A group of 83 patients, all of whom did not perfectly qualify for the procedure for various reasons (such as the disc herniation was too large, or they had too much degeneration), were also part of this investigation as there own subgroup. Outcomes from their procedure was only 49.4%. Of the patients who succumb to open discectomy, nearly 70% had an unrecognized sequester of free disc fragment.
In A very large observational study, Maroon and Allen [35] examined the outcomes of 1,054 patients who had undergone APLD procedures at 35 US hospital facilities. At follow-up (no time point was given), 82.9 of them were considered to have had a successful result by both the treating physician and the patient. Also interesting was the fact that on average 2.4 g of nucleus pulposus material was removed from the disc. Of all those patients, there were only three postoperative complications: two patients came down with discitis (disc infections) and one patient came down with the muscle hematoma. Therefore, the overall complication rate of this study was 0.002%.
In the most recent, even larger, multi-hospital study [28] of 1582 APLD patients, in which a modified instrument was used, touted a success rate of 83% at one year follow-up. Nine patients suffered discitis which brought the complication rate to 0.06%.
THE EVIDENCE:
The level of quality scientific evidence given to support APLD for the treatment of contained disc herniations, in accord with criteria described by the USPSTF [98], was opined to be Level II-2 for short and long-term relief of pain and suffering. Unfortunately, the included randomized controlled trials were unable to bump this up to a higher level of support.
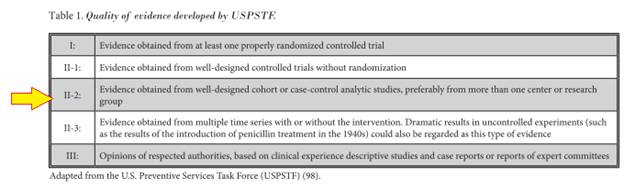
The official recommendation for the procedure, which was based on Guyatt et al’s (99) criteria, was 1C / strong recommendation.
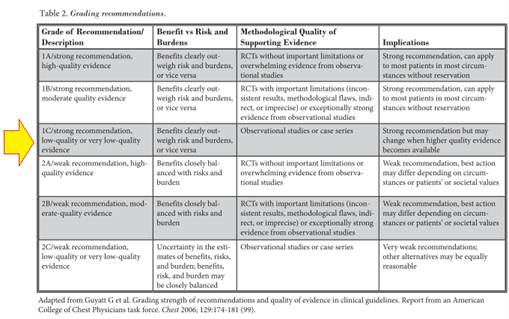
DISCUSSION AND CONCLUSION:
After their massive review of the medical literature database of quality medical investigations on the subject of automated percutaneous lumbar discectomy (APLD), this well-respected team of researchers concluded:
| "It is postulated that for patients with contained disc herniation, percutaneous mechanical disc decompression with APLD may be the best choice." |
With regard to safety, the authors stated, "APLD is considered safer than microdiscectomy since it utilizes the Nucleotome probe as the primary instrument for the decompression, limiting the amount of times that the physician needs to enter the disc space for the removal of nucleus pulposus."
They also opined, "Almost definitely, microdiscectomy is more invasive than APLD." This was because microdiscectomy uses manual instruments that may need to reenter the disc several times.
With regard to another well researched and efficacious treatment for small contained disc herniation, chymopapain injection (which is illegal in the United States), the authors stated, "the effectiveness of APLD appears to compare favorably with the results of chymopapain injection and open discectomy, even though it is very difficult to draw conclusions as these assumptions have not been proven in randomized controlled trials."
The authors closed by stating, "APLD… may provide appropriate relief in properly selected patients with contained disc herniation. APLD is a safe procedure with minimal complications."
My COMMENTS:
The only thing that was seemingly shied away from was the horrible results of the two randomized controlled trials that had enough scientific evidence to make it into this systematic review. The first ones poor patient outcome [53] can certainly be explained by the loose patient-entry criteria. But the second one [51] corrected for the mistakes of the first by having a strict patient entry criteria; however, the outcome was still poor, for the success rate was only 29% as compared to an 80% success rate for the control (microdiscectomy). That was a little scary for me.
Another salient point to discuss and consider is cost: While this minimally invasive procedure is much less expensive than open discectomy, it (Like all of these minimally invasive procedures) is not covered by insurance (Blue Cross/Blue Shield) or Medicare/Medicaid because it is considered "investigational."
The Bottom line in my humble opinion is that if you're rich, APLD is probably worth a try; however, it is only for a very select group of patients. More specifically, for patients who have a small, central-paracentrally located, contained disc herniation (<6mm or that occupies no more than 1/3 the central canal size); who do not have a full thickness, grade 5 annular tear; or who do not have disc-height loss secondary to degenerative changes. furthermore, 3 to 6 months of physical therapy must be tried without success, before you pull the trigger.
REFERENCES:
1) Gibson JNA, Waddell G. "Surgical Interventions for lumbar disc prolapse. Cochrane Database Syst Rev 2009; (1): CD01350.
10) Carragee EJ, et al. "Can MR scanning in patients with sciatica predict failure of open limited discectomy? Proceedings of the international Society for the study of lumbar spine (ISSLS) Scotland, June 2001.
11) Ng LC, Sell P. 'Predictive value of the duration of sciatica for lumbar discectomy. A prospective cohort study.'J Bone Joint Surg Br. 2004 May;86(4):546-9.
12) Dewing CB, et al. "the outcome of lumbar microdiscectomy in a young, active population: correlation by herniation type and level." Spine 2008; 33:33-38.
13) Carragee EJ, et al "Clinical outcomes after lumbar discectomy for sciatica: The effects of fragment types and annular competence" J Bone Joint Surg Am - 2003; 85(1):102-108
17) Onik G, et al. "percutaneous lumbar discectomies using a new aspiration probe." AJNR 1985; 6:290-293.
18) Onik G, et al. "percutaneous lumbar discectomy using a new aspiration probe: porcine and cadaver model." Radiology 1985; 155:251-252.
19) Bonaldi G. "automated percutaneous lumbar discectomies: technique, indications, and clinical follow-up in over 1000 patients." neuroradiology 2003; 45:735-743.
22) Onik G, Mooney V, Helms C, et al. "automated percutaneous discectomy: a prospective multi-institutional study. Neurosurgery 1990; 226:28-232.
28) Teng GJ, et al. “automated percutaneous lumbar discectomy: a prospective multi-institutional study." J Vasc Interv Radiol 1997; 8:457-463.
35) Maroon JC, Allen AC. "a retrospective study of 1054 APLD cases: a 20 month clinical follow-up at 35 US centers." J Neurol Orthoop Med Surg 1989; 10: 335-337.
51) Chatterjee S, Foy PM, Findlay GF. “Reporting a controlled clinical trial comparing automated percutaneous lumbar discectomy and microdiscectomy in the treatment of contained lumbar disc herniation." Spine 1995; 20:734-738.
53) Revel M, et al. “automated percutaneous lumbar discectomy versus chemonucleolysis in the treatment of sciatica: a randomized multi-center trial." Spine 1993; 18:1-7.
57) Hoppenfeld S. “percutaneous removal of herniated lumbar discs: 50 cases with 10 year follow-up periods.” Clin Orthop 1989;238:92-106.
58) Kambin P, Schaffer JL. “Percutaneous lumbar discectomy: review of 100 patients and current practice." Clin Orthop 1989; 289:24-34.
60) Vaccaro AR, Bono CM. “minimally invasive spine surgery.” (2007) In: Informa Healthcare USA, Inc. 52 Vanderbilt Ave., New York, NY 10017.
61) Singh V, Manchikanti L, Helm S, Hirsch et al. “Percutaneous Lumbar laser Disc Decompression: a Systematic Review of Current Evidence." Pain physician 2009; 12:573-588.
62) Marron JC, Onik G, Sternau L. “percutaneous automated discectomy: a new approach to lumbar spine." Clin Orthoop 1989; 238:64-70.
63) *Kloth DS, Singh V, et al. "Percutaneous disc decompression. In: Manchikanti L, Singh V (eds). Interventional Techniques in chronic Spinal Pain. ASIPP Publishing, Paducah, KY, 207; pp 601-622.
64) Koes BW et al. "Efficacy of epidural steroid injections for low back pain and sciatica: a systematic review of randomized clinical trials." Pain 1995; 63-279-288.
65) West S, et al. "Systems to rate the strength of scientific evidence, evidence report, technology assessment number 47." AHRQ Publication No. 02E016. Rockville, MD: Agency for Healthcare Research and Quality, 2002. www.thecre.com/pdf/ahrq-system-strength.pdf
66) Revel M, Payan C, et al. "automated percutaneous lumbar discectomy versus chemonucleolysis in the treatment of sciatica. A randomized multi-center trial." Spine 1993; 18:1-7.
67) Chatterjee S, Foy PM, Findlay GF."Report of a Controlled Clinical Trial Comparing Automated Percutaneous Lumbar Discectomy and Microdiscectomy in the Treatment of Contained Lumbar Disc Herniations." Spine 1995; 20:734-738.
86) Manchikanti L, Hirsch JA, Smith HS. “Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: Part 2: randomized controlled trials.” Pain physician 2008; 11:713-775.
87) Manchikanti L, et al. “evidence-based medicine, systematic reviews, and guidelines in interventional pain management: part three: systematic reviews and meta-analysis of randomized controlled trials." Pain physician 2009; 12:35-72.
88) Manchikanti L, Singh V, et al. “evidence-based medicine, systematic reviews, and guidelines in interventional pain management: part four: observational studies. Pain physician 2009; 12:73 – 108.
89) Manchikanti L, Singh V, et al. “effectiveness of thoracic medial branch blocks in managing chronic pain: a preliminary report of randomized, double-blind controlled trial:NCT00355706. Pain physician 2008; 11:491-504.
90) Manchikanti L, Singh V, et al. “cervical medial branch blocks for chronic cervical facet joint pain: a randomized double-blind controlled trial with one-year follow-up." Spine 2008; 33:1813 – 1820.
91) Manchikanti L, et al. “lumbar facet joint nerve blocks in managing chronic facet joint pain: one-year follow-up of randomized double-blind controlled trial: clinical trial NCT 00355914.” Pain physician 2008; 11:121-132.
92) Manchikanti L, Cash KA, et al. "preliminary results of randomized, equivalent trial of fluoroscopic caudal epidural injections in managing chronic low back pain: part one. Discogenic pain without disc herniation or radiculitis.” Pain physician 2008; 11:785-800.
93) Manchikanti L, Singh V, et al. “preliminary results of randomized, equivalents trial of fluoroscopic caudal epidural injections in managing chronic low back pain; part two. Disc herniation and radiculitis." Pain physician 2008; 11:801-815.
94) Manchikanti L, Singh V, Cash KA, et al. "preliminary results of randomized, equivalents trial of fluoroscopic caudal epidural injections in managing chronic low back pain: part three. Postsurgical syndrome. Pain physician 2008; 11:817-831.
95) Manchikanti L, et al. “preliminary results of randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: part four. Spinal stenosis. Pain physician 2008; 11:833-848.
98) Berg AO Allan JD. “Introducing the third US preventative service task force." Am J Prev Med 2001:20;S3-S4.
99) Guyatt G, et al. "Grading Strength of Recommendations and Quality of Evidence and Clinical Guidelines. Report from an American College of chest physicians task force." Chest 2006; 129:174-181.
126) Onik G, Helms CA. "automated percutaneous lumbar discectomy." AJR Am J Roentgenol 1991; 156:531-538.
156)
301) Kambin P, Gellman H, “percutaneous lateral discectomy of the lumbar spine: a preliminary report." Clin Orthoop 1983; 174:127-132.
302) Hijikata S. “percutaneous nucleotomy. A new concept technique and 12 years experience." Clin Orthoop Relat Res 1989; 238:9-23.
304) Onik G, et al. “percutaneous lumbar discectomy using a new aspiration probe." AJR 1985; 144:1137 – 1140.
305)* Onik G, Maroon J, Helms C. "automated percutaneous discectomy: preliminary experience." Acta Neurochir Suppl (wien), 1988; 43:58-62.
314)*book reference: Onik GM, Helms CA, et al. “percutaneous lumbar discectomy using an aspiration probe." AJRN 1985; 6:290-293.
Top
Copyright © 2002 – 2012 by Dr. Douglas M. Gillard DC
|

