The Sciatica Page |
 |
“Thou cold sciatica, cripple our senators, that their limbs may halt as lamely as their manners.” – William Shakespeare, 1564.
In order to achieve the best understanding of the contents on this page, it would behoove the reader to first visit the Disc Anatomy Page and then the MRI Page, for I will make the assumption that you know the basics of anatomy and MRI reading.
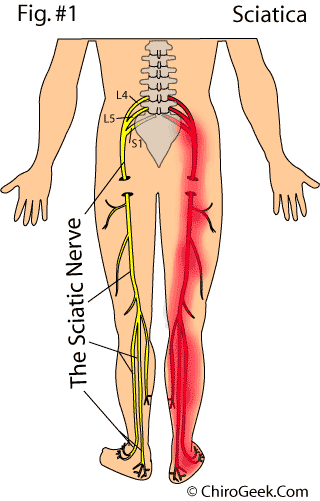 As you may have gathered from the Shakespearian quote above, sciatica has been torturing mankind for a very long time. No doubt since the time that humans evolved into upright creatures, which in turn greatly increased the biomechanical stresses upon the disc which led to its occasional failure. Although there has been biblical mention of it, [6] the first detailed report of sciatica was made in 1550 B.C. as written in the medical text, The Edwin Smith Surgical Papyrus. [10]
As you may have gathered from the Shakespearian quote above, sciatica has been torturing mankind for a very long time. No doubt since the time that humans evolved into upright creatures, which in turn greatly increased the biomechanical stresses upon the disc which led to its occasional failure. Although there has been biblical mention of it, [6] the first detailed report of sciatica was made in 1550 B.C. as written in the medical text, The Edwin Smith Surgical Papyrus. [10]
Although we have certainly come a long way with regard to its diagnosis and treatment, [63] by no means do we completely understand the pathophysiology of sciatica, and whether or not our modern treatment interventions are superior to those of old, remains equivocal.
Sciatica, which often strikes without cause in the fifth decade of life, [22] is a symptom of burning, shooting, stinging, or numbing pain that typically radiates (shoots) from the lower back, down the entire lower extremity, often ending up in the foot (figure 1, red). [7] These symptoms can be aggravated by lifting, bending, sitting, standing, and walking, and are generally classified as a neuropathic pain; therefore, they are akin to the pain of diabetic neuropathy and postherpetic neuralgia. [82]
As we shall discuss further below, sciatica occurs when one of the nerve roots of a motion segment (a motion segment consists of two adjacent vertebrae and the interconnecting disc and soft tissue) becomes irritated which can result in severely disabling pain that sometimes is very difficult to get rid of. Although it usually occurs at the L4/L5 or L5/S1 level (motion segment), problems at L1/L2, L2/L3, and L3/L4 certainly do occur and may present with atypical findings on examination.
Sciatica may also be called radicular pain, radiculopathy, lower extremity pain, lower limb pain, and "leg pain." Please note that with the exception of "leg pain," I will use these terms interchangeably; however, the correct medical term is radicular pain.
Approximately 7 out of every 1000 people (0.07%) are struck down with sciatica each year, [3,5,33] and 5% of the population will experience this burning pain at least once during their lifetime. [14]
The good news is that for those affected by sciatica for the first time and who did not require hospitalization, 60-80% of them will completely recover within 6 weeks. [5,49] However, that "complete recovery" is occasionally short-lived, for 10-15% of the people who recovered will suffer recurrent bouts of sciatica throughout their lives. [21]
The clinical picture for patients with severe sciatica that is bad enough to necessitate hospitalization is even more ominous. Specifically, in 1999 Balague et al. [22] published the results of their sciatica study that followed 82 consecutive patients that were hospitalized for true below-the-knee radicular pain. While in the hospital, all of the patients were treated with medication, epidural steroid injections, physical therapy, and bed rest. At the one-year follow-up evaluation, only 28.4% of the people were pain-free and without disability. Even the 33% that tried surgery during the study-period fared no better. [22]
Therefore, for patients who suffer true radicular pain (especially if radiculopathy was present or if hospitalization was necessary), the road to complete recovery is filled with uncertainty.
Beside having bad genetics for disc building material, which is probably the number-one risk factor for disc herniation-associated sciatica, your occupation may significantly increase the chances of developing disc herniation-associated sciatica. Specifically, research has indicated that performing heavy manual labor and, ironically, sedentary work are the two types of employment most frequently associated disc-herniation-related sciatica. [195] This finding was confirmed in other investigations that demonstrated that frequent heavy lifting, [193,194] frequent twisting and bending, [193,194] exposure to vibration (such as in operating a jack hammer), [192,193] and sedentary activity [190,192,195] all increase the risk of developing sciatica.
Research has also demonstrated that the type of occupation least associated with the development of sciatica were occupations that required a combination of sitting, standing, and moderate physical activity. [95]
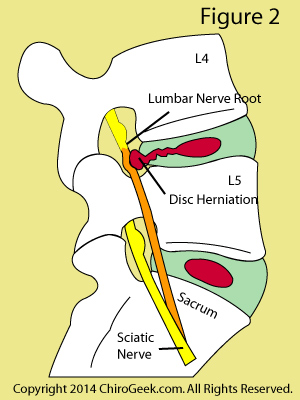 In layman's terms, radicular pain occurs when one of the sciatic nerve roots (either L4, L5, or S1) becomes compressed and inflamed, typically from either a disc herniation, or a condition called stenosis.
In layman's terms, radicular pain occurs when one of the sciatic nerve roots (either L4, L5, or S1) becomes compressed and inflamed, typically from either a disc herniation, or a condition called stenosis.
Let's talk more about each of these conditions.
Figure 2 demonstrates the most common cause of sciatica in middle age folks: a disc herniation. Note that an annular tear has developed within the L4 disc and has allowed the nucleus pulposus (red) to escape posterolaterally and compress the L4 exiting nerve root (orange). The typical symptoms of such a herniation include a strong burning pain in the L4 dermatome; i.e., the outside of the thigh and inside of the leg and foot. Neurological findings may include patellar reflex change, and a weakness in the muscles that dorsiflex (raise up) the foot and big toe. In severe cases, the patient may have great trouble walking and may even trip over their affected foot, which is a condition called foot drop.
I have previously explained in great detail how a disc herniation may cause sciatica and will not entirely repeat myself here. Please go to the forthcoming link in order to really dig into this topic. (read more)
In a nutshell, there are two factors that must be in effect in order for a disc herniation to cause sciatica: (1) there typically, but not always, [89] has to be compression (or lease contact) of the nerve root by the disc herniation, and (2) an inflammatory process must have developed in and around the nerve root, because of that compression. [64,65]
Let's talk a little bit more about these two factors.
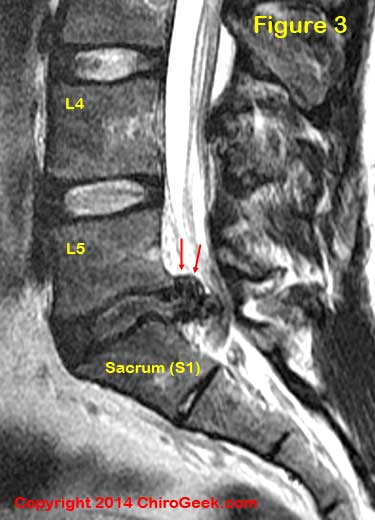 Although it is well known that nerve root compression alone does not always result in sciatica, [54,56,64] prolonged physical compression of sufficient magnitude, however, will certainly set the stage for its development secondary to an interference with the nerve root's circulatory system.
Although it is well known that nerve root compression alone does not always result in sciatica, [54,56,64] prolonged physical compression of sufficient magnitude, however, will certainly set the stage for its development secondary to an interference with the nerve root's circulatory system.
In order to understand this concept of circulatory impairment, you are going to have to learn a little bit about nerve root anatomy and physiology.
Like any living tissue, the lumbar nerve root, which is made of motor, sensory, and nociceptive (pain) axons, needs oxygen, nutrients, and waste removal in order to survive. Therefore, each nerve root has its own vein, artery, and rudimentary lymph vessel in order to satisfy these basic requirements of life. The cerebral spinal fluid that percolates around each nerve root within the dural sleeve also affords such nourishment. [52]
Unlike the tough peripheral nerves (nerves found outside of the spinal column), lumbar nerve roots are vulnerable to compression. [52,55] This is because they lack the extra layers of protection that peripheral nerves have—they don't have an epineurium (for the most part) or a perineurium (at all). [56]
If the nerve root is compressed by a disc herniation in just the right spot for just the right amount of time, then its circulatory system will begin to fail as the venous blood flow becomes restricted and backs-up, a condition called venous stasis. This failure begins to kill the tiny axons of the root, for their supply of oxygen and nutrients are cut-off, and their acidic waste products can't be removed from the area. The percolation of nutrient-giving cerebrospinal fluid is also impaired and no longer bathes the nerve root in a symmetrical fashion.
As the axons die, they will begin to spontaneously fire-off (ectopically discharge) their signals to the brain and/or periphery. If the sensory axons, which include the unmyelinated pain-carrying c-fibers, ectopically discharge, then the brain will receive signals of pain (nociceptive signals) and a sensation of pins and needles (sensory disturbance). The brain then erroneously interprets these signals as coming from the origin of the axons, i.e., the lower extremities, and not from the actual point of compression, i.e., the low back. If the motor axons are affected, then the muscles of those axons will experience pain, cramping, twitching and even atrophy.
*It is important to understand that the compression, inflammation, and damge of the lumbar nerve root do not cause low back pain; these factors result in sciatica. What does cause the back pain? The annular tear that spawned the disc herniation is usually responsible for low back pain associated with disc herniation. (read more) However, fractures within the posterior arch, inflammation of the facet joints, inflammation of the sacroiliac joints, and, rarely, problems with the piriformis muscle may also cause low back pain.
If the nerve root comes in contact with a disc herniation that happens to be leaking "evil' biochemicals called cytokines (especially one called tumor necrosis factor-alpha), then inflammation may well begin within and around the nerve root, via an inflammatory response and/or an autoimmune response. [57,58]. Again, I have covered this topic in detail on the disc herniation page. (learn more)
The bottom line is this: once a nerve root gets inflamed, it becomes hypersensitive to compression; therefore, even the slightest compression may be enough to begin the vicious cycle of inflammation, compression, and sciatica. [64]
In order to make things even more confusing, there is a small amount of research that demonstrates radicular pain can be caused by a leaking annular tear without the component of compression or even nerve root contact. [89]
Specifically, in 2007 Peng et al. [89] published the results of a unique investigation (a Chinese study) in a reputable medical Journal that reported CT discography results of 42 patients who were suffering low back pain and sciatica yet had no indications of disc herniation or nerve root compression on MRI. Discography results demonstrated that all patients demonstrated a full-thickness posterior annular tear, usually on the same side as the sciatica, on CT follow-up and had their lower extremity pain and lower back pain re-created by pressurization of the disc (concordant pain). They hypothesized that biochemicals had leaked out of the disc and inflamed the adjacent nerve roots, which in turn resulted in radicular pain without nerve root compression. Interestingly, 76% of these patients had a positive EMG test. [89] *This is a very rare study and should be taken with a grain of salt.
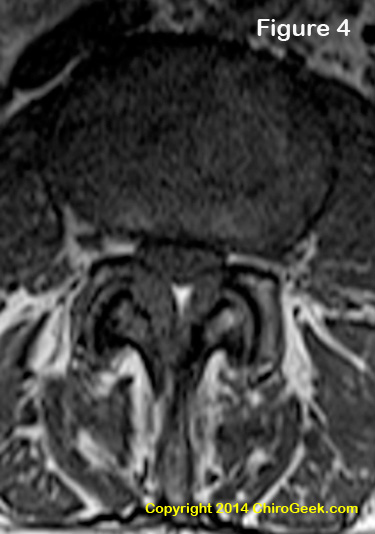 Stenosis is a condition that occurs when one or more of the bony holes within the motion segment (i.e., the vertebral canal, lateral recesses, and neuroforamina) become narrowed, and the neural structures within them (i.e., the thecal sac, cauda equina, traversing nerve roots, and exiting nerve roots) get compressed (squeezed) or even crushed by the narrowing.
Stenosis is a condition that occurs when one or more of the bony holes within the motion segment (i.e., the vertebral canal, lateral recesses, and neuroforamina) become narrowed, and the neural structures within them (i.e., the thecal sac, cauda equina, traversing nerve roots, and exiting nerve roots) get compressed (squeezed) or even crushed by the narrowing.
Figure 4 is an axial T1-weighted MRI image through the L3 disc space that demonstrates moderate central stenosis secondary to severe ligamentum flavum thickening and disc bulging that resulted in the symptoms of neurogenic claudication in this patient. Note that the thecal sac is completely surrounded by and squeezed the disc bulge and ligamentum flavum thickening. Can't see it? Click here for some help.
Such nerve root compression, as discussed above, may compromise nerve root circulation, which in turn leads to ectopic axon firing and subsequent lower extremity pain and dysfunction.
There are two different types of stenosis that may occur in either isolation or together: central stenosis and lateral stenosis.
Central Stenosis occurs when the central portion of the vertebral canal looses its anterior-posterior diameter to such an extent that compression of the thecal sac and lumbar nerve roots (cauda equina and traversing nerve roots) occurs. Although such compression may cause radicular pain secondary to compression of the traversing nerve root injury, most of the time it causes a condition called neurogenic claudication from compression of the cauda equina.
Neurogenic claudication, which is rarely seen before the sixth decade of life, [84] is a bazaar condition that strikes a patient during walking (ambulation). Here's the typical scenario: after walking a certain distance, the patient begins to experience pain, weakness, and tiredness in one or both lower extremities (usually both). These symptoms eventually gets so severe that he or she is forced to sit down and rest. After the rest, the leg symptomatology disappears and the patient may resume walking until the scenario plays out again. To speed the recovery, the patient may need to bend forward waste (such as in pushing a shopping cart) which is called "shopping cart sign." [84] Neurogenic claudication is thought to occur because walking places an increased demand for nerve root oxygen and nutrients, a demand that cannot be met because the vessels of the nerve root are compressed and will not allow for the needed blood volume increase. Therefore, the previously described train of events occurs (venous stasis, axon stress, ectopic discharge and pain) which ultimately leads to the symptomatology of neurogenic claudication. [66,83,84]
Lateral Stenosis occurs when the regions lateral to the central portion of the vertebral canal (i.e., the lateral recesses and neuroforamina) lose their anterior-posterior diameter and compression of the traversing and exiting nerve roots occurs. Such compression virtually never causes neurogenic claudication; however, it does commonly cause radicular pain.
Let's talk about what actually causes the bony narrowing of the motion segment holes.
Stenosis is typically caused by degenerative change (arthritis) within and around the structures that border the motion segment holes. Via mechanisms to complicated to discuss here, such degenerative change may result in bony thickening (eburnation, arthrosis or sclerosis), ligamentous thickening, and even loss of motion segment stability (i.e., degenerative spondylolisthesis), [62] all of which can narrow the dimensions of the vertebral canal, lateral recess, and neuroforamina.
Other common causes of stenosis include segment instability, often secondary to spondylolysis, isthmic spondylolisthesis, or posterior arch malformation; malposition, secondary to fixed spondylolisthesis; scoliosis; and facet cysts. Rarely, tumors or cysts of the neural tissue can cause stenosis; however, these and other rare causes will not be discussed on this page.
Let's talk a little more in detail about some of the more common causes of stenosis.
I have dedicated an entire page to the subject of Spondylolysis and Spondylolisthesis. Please visit that page for more information.
In a nutshell, if a fracture or defect within the pars interarticularis occurs, such as in isthmic spondylolisthesis, then the superior vertebra of the motion segment may become unstable and in fact slide forward (anteriorly translate) which in turn jams either the beak of the fracture or the facet into the lateral recess and/or neural foramen. Such a condition narrows those holes and causes lateral stenosis. This condition typically does not cause central stenosis, for the central canal usually gains anterior-posterior dimension from the anterior translation.
Degenerative spondylolisthesis, on the other hand, is often associated with severe bony thickening of the posterior arch and facet joints, the latter of which fail and allow for anterior translation. Because of this thickening, degenerative spondylolisthesis is usually associated with central stenosis, which may or may not be associated with lateral stenosis.
Scoliosis is a condition that occurs when the normally straight spine becomes crooked from and anterior-posterior perspective. Such abnormal curves destroy the normal biomechanics of the spine and place tremendous axial load in places that are not designed to handle such stress, especially the neural foramen. With the passage of time, the neural foramen at the concavity of the curve may collapse which results in lateral stenosis, which of course can compress both the exiting and traversing nerve roots.
There are other causes of sciatica that are considered rare, and I am not going to get into them other than to mention their names: isolated biochemical nerve root irritation, discogenic referred pain syndrome, facet cyst, nerve root tumor (Tarlov cyst), nerve roots stretch injury, piriformis syndrome, epidural venous plexus compression, infection, cancer, blood clots (deep vein thrombosis), and possibly a phenomenon associated with recombinant human bone morphogenetic protein-2.
I would be more than happy to discuss these other causes with you during one of my Coaching Sessions.
You can't have sciatica unless you have complaints of lower extremity pain that is usually described as an unpleasant burning or tingling sensation, a deep throbbing pain, or a shooting electrical pain. The scope of true radicular pain usually involves the entire lower extremity as the pain travels from the buttock, into the posterior or posterolateral thigh, crosses the knee, passes through either the anterolateral or anteromedial leg, and terminates in the lateral, medial, or dorsum of the foot.
Sciatica is usually isolated to one lower extremity; however, it is not uncommon for it to occur in both lower extremities (bilaterally), especially in patients with central stenosis. [62]
Although true sciatica typically radiates below the knee, high disc herniations or stenosis (i.e., herniations or stenosis at the L1/L2, L2/L3, or L3/L4 level) can cause true radicular pain that is isolated above the knee, in the anterolateral, anterior or medial thigh and maybe in the groin. However, in a very small study (n=10), the distribution of this pain has been reported in 90% of the patients with either L2 or L3 disc herniation to affected both the L5 and S1 dermatome. [86]
Confusingly, conditions such as facet syndrome, sacroiliac syndrome, and discogenic pain syndrome can refer pain into the lower extremity which may cloud the diagnosis of disc herniation or stenosis-related radicular pain. However, such referred pain rarely passes below the knee and will never, in and of itself, results in radiculopathic findings such as muscle atrophy, loss of sensation, or deep tendon reflex change.
Therefore, just because you have sciatica, it doesn't mean that you have true radicular pain (radiculopathy) as a result of a disc herniation or stenosis. More testing is needed to make that determination.
Although key physical examination findings, which includes a postive straight leg raise test, diminished muscle strength, loss of sensory perception, and deep tendon reflex change, are traditionally used by clinicians to make the diagnosis of disc herniation-related or stenosis-related radicular pain, recent medical literature has cast serious doubt their ability to accurately diagnose a symptomatic disc herniation. [71,79,85,87]
On the other hand, if the patient's radicular pain falls nice and neatly into one of the regions of skin that is specifically supplied by a single spinal nerve (these regions of skin are called dermatomes), then medical research has demonstrated this finding is quite predictive disc herniation. [85,86] However, such a finding is still not nearly as accurate as positive MRI [90] or EMG [92] findings, both of which we will discuss farther below.
As a side note, although dermatome pain is useful at predicting the presence of the disc herniation, it is not very useful at predicting the exact level of the disc herniation. [71]
Magnetic resonance imaging (MRI) is by far the most accurate diagnostic tool (it has high sensitivity and specificity) for making the diagnosis of disc-herniation-related radicular pain, [90] and it does a pretty good job diagnosing stenosis-related radicular pain too; however, computed axial tomographic (CT) is better at detecting stenosis than MRI. (Learn more about MRI)
 Another important test that should not be overlooked for patients with lower limb pain is electromyography (EMG), which has been found to have high specificity (i.e., a low false positive rate) for detecting radiculopathy. [92]
Another important test that should not be overlooked for patients with lower limb pain is electromyography (EMG), which has been found to have high specificity (i.e., a low false positive rate) for detecting radiculopathy. [92]
Electromyography, which should be accompanied by a nerve conduction velocity study (NCV), has the unique ability to detect nerve-root-level axon damage (i.e., damage to the tiny nerve fibers that makeup the nerve root) and will really finalize the diagnosis of disc-herniation- or lateral stenosis-related radicular pain if found to be positive.
This test also has the ability to differentiate between disc-herniation-related sciatica and other causes of sciatica, such as diabetes and herpes zoster, as well as differentiate between acute and chronic radicular pain.
*If the EMG is positive (i.e., there are indications of denervation {axon death} via increased insertional activity, positive sharp waves and/or fibrillation potentials), then the patient is said to have radiculopathy, which means that the radicular pain has most likely occurred from the dead and dying axons within the compressed and inflamed nerve root.
One of the pitfalls of EMG/NCV, however, is its low sensitivity which means that it misses a lot of true cases of radiculopathy (i.e., a high false-negative rate). However, because of its very high specificity [92]—when it finds radiculopathy, you really have radiculopathy (very low false-positive rate)—it is definitely an important tool for really nailing down the diagnosis of disc herniation-related radicular pain and should always be ordered for a patient with lower extremity pain and positive neurological findings. In fact, the 2014 study by Savage et al. [88] reported that neither patient history nor key examination findings could outperform EMG when it came to picking out which patients had true radiculopathy. [88]
*The test is also time-sensitive and should never be performed less than four weeks from the onset of the radicular pain. In other words, if it is performed too early, it will almost always be completely normal, for it takes time for the damaged axons to affect the muscles that will be tested by the procedure. (**Case and point: I went a pain management doctor (a physiatrist) here in the bay area for the purpose of medication refill, and to my surprise she wanted to perform an EMG/NCV on me, notwithstanding the fact that my sciatica flared-up had only just happened one weeks prior! I politely declined and educated her {which of course annoyed her greatly}. Did she not know the physiology of EMG/NCV? Or was she just trying to increase her bottom line? You have got to know this stuff people!)
Here's another EMG pitfall: the EMG/NCV study does not test the nerves that actually carry the signals of pain (i.e. the small non-myelinated C-fiber); it only tests the large myelinated motor nerves, which is probably why it has such low sensitivity.
Okay, I can hear you saying, "What is the point of suffering through this painful study when it doesn't even test the pain-carrying nerve fibers?" The smaller pain-carrying nerve fibers are in very close proximity to the larger myelinated nerve fibers which are tested. Specifically, all of the flavors of nerve fibers are are tightly bundled within the exiting or traversing nerve root, which in turn are encased within the dural sleeve. Therefore, as medical research has shown, if the large myelinated fibers are damaged, then you can bet your bottom dollar that the small non-myelinated pain fibers are damaged as well. [100] *There are, however, some conditions that only affect the small non-myelinated nerve fiber, but that is beyond the scope of this page.
*Note: I have not updated this section of the sciatic page just yet, but am currently working on it.
[Surgery Timing | Surgery Indications | Treatment Guidelines | Epidural Steroid Injections ]
Without question, the best treatment for non-complicated, non-emergency sciatica is passage of TIME. Even the worst cases of sciatica may spontaneously resolve with time (albeit a long time), and even some very large disc extrusions may completely be reabsorbed by the body, [23] thereby decompressing the nerve root naturally. BUT, if you are a candidate for surgery and are not improving with care at all, then delaying the surgery seems to decrease your chances for a successful surgical out come. [305]
The American Academy of Orthopedic Surgeons and Alf Nachemson – who is the number one spine researcher in the world - (18) recommends the following conditions be met before decompressive surgery is offered:
#1 |
Functional incapacitating pain in the leg, extending below the knee with a nerve root distribution. |
|---|---|
#2 |
Nerve root tension signs (positive straight leg raising test) with or without neurologic abnormalities, fitting the radiculopathy. |
#3 |
Failure of clinical improvement after 4-8 weeks of conservative treatment, |
#4 |
Confirming imaging study: abnormal myelogram, computed tomogram (CT), or magnetic resonance imaging (MRI) correlated to the physical signs and distribution of the pain. |
The only ABSOLUTE indication for surgical intervention, however, is if the patient develops a loss of bowl or bladder control (cauda equina syndrome); if the patient develops severe progressive motor loss in the lower limbs, such as foot-drop; or if the patients has severe debilitating pain.
Several high quality outcome assessment investigations have shown that even patients with confirmed radiculopathy (nerve root damage) that meet the 'four criteria' for surgery often do 'well' with non-surgical treatment (77 - Saal & Saal); furthermore, although patients who undergo discectomy seem to get better faster (rarely 100% better), in the long-run (4 to 7 years) the non-surgical patients obtain nearly the same improvement in terms of pain relief and functional outcome. (78 - Weber)
SURGERY TIMING: When to "Pull the Trigger" and Try Surgery?
It is generally agreed upon, assuming the patient is NOT improving, that one should not wait too long before undergoing decompression via discectomy. The the experts can't seem to agree upon is how long the patient should be allowed to suffer. The forthcoming investigations all yield different opinions on when surgery should be performed; however, they all agree that a suffering patient should not wait longer than one year. The average time is currently 4.6 months, so waiting longer than this might decrease your chances of having a successful discectomy. I would seem to be living proof of this, i.e., I waited 15 months and my surgery failed miserably.
Research papers: |
Year (foot note) |
Max length of conservative care; after which microdiscectomy should occur: |
|
|
|
| McCulloch | 1996 (53) | 3 months |
Postacchini F. |
1999 (14) |
6 months |
Dvorak J, et al. |
1988 (11) |
4 months |
Hurme M. & Alaranta H. |
1987 (12) |
2 months |
| Ng LC & Sell P. | 2004 (66) | < 12 months |
Rothoerl RD , et al. |
2002 (26) |
2 months |
| Nygaard et al. | 2000 (50) | 8 month |
| Jansson et al. | 2008 (51) | 6 months |
Dauch WA , et al. |
1994 (10) |
6 weeks |
Expert Average: What is the longest a patient should wait--assuming conservative care is failing--before having microdiscectomy or open discectomy? |
|
months |
I’ve read a tremendous amount of research and picked-the-brain of many a doctor during my own battle with sciatica. Based on my research and personal experience I've developed some recommendations for the treatment of sciatica (*remember, these are to be discussed with your primary treating physician and this is not to be construed as medical/car practical advice):
| Start anti-inflammatory medication such as Naproxen, Ibuprofen, and Celebrex, as soon as possible. The Association between sciatica and the development of chronic pain is becoming stronger and stronger, so anti-inflammatory measures are a must--be careful of your stomach, however, and don't try to go on a long-term course of these without doctor supervision! He/she may prescribe a proton pump inhibitor to help you tolerate these better. And of course very long-term use can be hard on the liver and kidneys, so your primary care physician should order blood testing to monitor these organs. |
| Take ½ aspirin per day to thin the blood. This may help prevent clotting and venous stasis within the of microvasculatory system within the delicate nerve root. It is believed that damage to this micro-circulatory system within the delicate nerve may lead to permanent axon death, scaring within the nerve root, a poorly functioning circulatory system around the nerve and in the nerve, chronic hypersensitivity of the nerve root and chronic pain. (Remember to check with your MD first!) |
| Get at least one transforaminal epidural steroid injection (aka: TFESI) performed under done under fluoroscopy at all suspected root levels. Don't monkey around with nerve root blocks which contain anesthetic only UNLESS they administer the steroid immediately afterwards or as part of the injection (which they usually do). See Below for more information. |
| Start conservative care promptly: this may include physical therapy, exercise, swimming, and perhaps traction treatments. Try and stay as active as possible without aggravating your pain to severely. Home traction devices and/or a slant board are also worth trying. |
| Try a 14 day course of oral steroids (prednisone) to help reduce inflammation that might be occurring in an area that was not reached by the epidural injections. *follow your doctor's orders exactly been using this medication for could damage your adrenal glands and cause other problems if you stop cold turkey (you need to taper off this medication). |
| At the three-month-mark, if you’re NOT IMPROVING, get an immediate neurosurgical opinion! You absolutely do NOT want to wait too long for an indicated surgery (especially if there is moderate to severe nerve root compression). Numerous research papers have found that your chances for obtaining a successful surgical result substantially decrease with the passage of time beyond three or four months (301-309). |
We know that it is not all about physical compression (via disc herniation) of the nerve root. It is well-established that an inflammatory process (sparked by biochemicals called cytokines) has just as much to do with the pain syndrome of sciatica. And the best way to stop the damaging inflammation cascade is at the first step in the cycle [membrane phospholipids --> Arachidonic acid has catalyzed by phospholipase A2 ( PLA2)]. More specifically, the injected cortisone will inhibit the enzymes PLA2, as well as COX2, which in turn will snuff out the damaging inflammatory cycle that most likely is occurring within the adjacent nerve roots.
Although many medical clinics are injecting corticosteroids epidurally (between the lamina), the evidence for this administration route is still lacking [48]. Therefore, I continue to suggest that epidural steroid injections be performed only transforaminally under the guidance of fluoroscopy.
Also, make sure that lidocaine (the stuff that deadens your nerves at the dentist office) is included with this steroid injection. This can be extremely diagnostic, for the lidocaine will immediately blot out the patient's pain if that is indeed the nerve root that is swollen and inflamed. The patient should notice almost immediate pain relief following the injection, which will initially wear off (much to the patient's chagrin) after several hours. Make sure the patient keeps a pain journal and give them a heads-up for that crucial few hours postinjection. The steroid component of the injection will kick in after a few days to relieve the pain again and this time the relief should be for a few months if not longer. But remember, it is the injection of that lidocaine and its effect for the first three hours that is diagnostically important for this procedure; the more permanent pain relief will occur because of the steroid in a day or two following the injection.
WHAT ARE MY CHANCES OF GETTING SCIATICA?
For the answer to this question, we must turn the giant population based survey studies which are mostly flawed with respect to sciatica, for they don’t differentiate between true root related sciatica, and fake sciatica (referred lower limb pains from the SI joint, intraspinal ligaments, and/or facet joints etc).
From these less specific studies we have learned that the life-time chance (prevalence) for developing ‘generic sciatica’ (sciatica of unknown cause) is about 35%; this number has been pretty well substantiated in other investigations as well (181,183-186). Over the course of each year, about 1% of the North American population will suffer an attack of sciatica (9); that equates to about 4.9 MILLION cases per year!
Only two groups of investigators has had the intelligence to address the life-time prevalence of true root-related sciatica: Heliovaara et al. found that 5% of men and 4% of women will develop nerve root-related sciatica at some point in there life (181). These numbers were later confirmed by Manninen P, et al in 1995 (182).
The differentiation between the life-time chances of generic sciatica (35%) and true root-related sciatica (5%) demonstrates that true root-based sciatica is a relatively rare phenomenon. (Don’t we feel special?)
There are no studies that have investigated the prevalence for ‘discogenic pain/sciatica’ in the population, but I’m guessing it to be around 1% or 2%.
WILL I RECOVER FROM SCIATICA? [ Pertinent Research outcome ]
The short answer: probably not 100% if your sciatica is a true radiculopathy confirmed by EMG and/ or severe.
Unlike lower back pain, sciatica has a more prolonged and less certain recovery period. For the general non-radiculopathic sciatica, 40% to 50% of those affected will completely recovery within 4 weeks (19,20), and 88% will recovery within 6 months (8); however, 5% to 10% will not recover and require surgical decompression (9).
For us patients with 'real' disc herniation-associated radicular pain (radiculopathy), the outcome is less favorable, as has been demonstrated by several well-written investigations: Only 37% will completely recovery from there radicular pain (sciatica) by 3.5 years! This number was derived by averaging out all the complete recovery patient from the major five outcome studies of our time. (here)
The Saal brothers’ 1989 outcome study is undoubtedly the best paper to date on the recovery-rates for radiculopathy-related sciatica: the Saals followed 58 patients who had confirmed disc herniation-induced radiculopathy (via MRI & EMG). They had all failed ‘passive conservative care’ and were put through the authors aggressive conservative care program, which included special exercises, medications, epidural steroid injection and physical therapy. After 2 ½ years, only 30% of the patients reported a full recovery and 10% were lost to surgery. The good news - if you’re not an athlete - is that 90% of the patients returned to their original work and recovered enough to at least be able to perform some limited recreational activities (77).
Atlas et al. followed 220 patients that were treated surgically for disc herniation-induced sciatica and 183 patients who were non-surgically treated for the same. At five years only 28% of the surgically treated patients reported that their pains were completely gone. The conservatively treated group faired even worse, in that only 12% reported a complete resolution of their symptoms (15).
Another outcome study by Nykvist et al. found that after five years of either surgical or non-surgical treatment; 82% of the non-surgically treated group and 62% of the surgically treated group still suffered with at least some degree of sciatica (17)!
Weber et al. studied the outcome for a group of 208 non-surgically treated patients, all of whom had “root symptoms” associated with sciatica. After one year, 30% of the patients had reduced capacity in work, and restrictions of leisure activity and only 50% reported no pain at work or leisure (19).
The Volvo Award Winning Weber Study:
In the only quality ‘randomized’ outcome study to date, Henrik Weber followed a group of 126 sciatica patients who were ‘randomly’ assigned to either surgery or non-surgical care. All of these patients were considered ‘boarder-line’ surgical candidates and were followed for over 10 years. Over that 10 year period, only 5% of the patients were lose, which gives this Volvo Award Winning study excellent predictive value. At four years post ‘treatment’, only 66% of the surgical patients were “completely satisfied” with their outcomes, versus only 52% of the conservatively treated group. At 10 years, only 58% of the surgical group were completely satisfied (some had worsened), and 56% of the conservative group were completely satisfied. At 10 years only 2% of both groups reported residual lower limb pains (sciatica), which was greatly improved from the 37% and 23% residual sciatica reported sciatica at 4 years (16).
The Balague Study of 1999: (126) This group of Swiss and American investigators study the outcomes of 82 'severe sciatica patients' - patients were hospitalized because of the severity of their sciatica. 33% of the patients were lost during the one year follow-up period to surgery, although they were still followed up. As for the recovery of this cohort, the authors stated the following, "...a substantial percentage of patients with acute severe sciatica had not recovered by 1 year after discharge." More explicitly, only 21 (29%) of the all 82 patients were "essentially" pain free at one year. Interestingly, only 74% of these patients had disc herniation on MRI, and only 62% had a positive EMG!
| Radiculopathy Outcome Studies: | Complete Recovery or Completely Satisfied with Outcome: |
|---|---|
| Saal & Saal. 1989 (77) | 30% (conservative) @ 2 ½ years |
| Atlas et al. 2001 (15) | 28% (surgical) 12% (conservative) @ 5 years |
| Nykvist et al. 1989 (17) | 38% (surgical) 18% (conservative) @ 5 years |
| Weber et al. 1993 (19) | 54% (conservative) @ 1 year |
| Weber. 1983 volvo award (16) | 66% (surgical) 52% (conservative) @ 4 years |
| Balague et al. 1999 (126) | 29% (surgical & conservative) @ 1 year. |
| Average % of Complete Recovery: (I took the average from all 6 investigations, also averaged surgical & conservative, when applicable, into one number before computing final number.) | ONLY 37% of sciatica patients, with or without surgery, recover completely or near completely (33% from conservative care and 40% from surgical care). |
REFERENCES:
1) Kortelainen P, et al. “Symptoms and signs of sciatic and their relation to the location of the lumbar disc herniation.” Spine – 1985; 10:88-92
2) Nitta H, et al. Study on dermatomes by means of selective lumbar spinal nerve root block. Spine 1993;18:1782-1786.
3) Anderson GBJ. "The epidemiology of spinal disorders." In: Fermoyer JW, ed. The adult spine. New York:Raven Press:1997
4) Igarashi T. et al. "Volvo Award Winner 2000: Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology: Molecular, histologic, and behavioral comparisons in rats." Spine 2000; 25:2975-80
5) Van de Velden J, de Bakker DH. Basisrapport: morbiditeit in de huisartsenpraktijk. Utrecht: Nivel;1990
6) Fardon D. “Biblical pain: Did Jacob have Sciatica?” Spine J 2002; 2(3):228
7) Bogduk N. "Clinical Anatomy of the Lumbar Spine and Sacrum. New York: Churchill Livingstone, 1997.
8) Hakelius A, “Prognosis in sciatica: a clinical follow-up of surgical and non-surgical treatment.” Acta Orthop Scand 1970; Suppl 129
9) Frymoyer J, “Back pain and sciatica.” N Engl J Med – 1988; 318:291-300
10) Breasted JH (1930) Edwin Smith Surgical Papyrus, in Facsimile and Hieroglyphic Transliteration and with Translation and Commentary, 2 vols. Chicago: University of Chicago Oriental Publications.
11) Ohnmeiss DD, et al. "Degree of disc disruption and lower extremity pain" Spine 1997; 22(14):1600-1605
12) Ohnmeiss DD, et al. "Relation between pain location and disc pathology: a study of pain drawings and CT/discography." Clin J Pain 1999;15:210-7.
14) Herkowitz HN, Dvorak K, Bell G, et al. "The Lumbar Spine-3rd edition." Lippincott Williams & Wilkins 2004; Philadelphia, PA
15) Atlas SJ, et al. "Surgical & nonsurgical management of sciatica secondary to a lumbar disc herniation: Five year outcomes from the Maine Lumbar Spine Study." Spine - 2001; 26(10):1179-1187
16) Henrik Weber, '1982 Volvo Award in Clinical Science' "Lumbar Disc Herniation: A controlled, Prospective Study with Ten Years of Observation." Spine - 1983; 8(2):131-140
17) Nykvist F, et al. "A prospective 5-year follow-up study of 276 patients hospitalized because of suspected lumbar disc herniation" Int. Disabil. Studies - 1989; 11(2):61-67
19) Weber H, et al. "The Natural Course of Acute Sciatica with Nerve Root Symptoms in a Double-Blind Placebo-Controlled Trial" Spine 1993; 18(11):1433-1438
20) Andersson GJB et al. “The intensity of work recovery in low back pain.” Spine 1983; 8:880-884
21) Biering-Sorensen F, Thomson C (1986) "medical, social and occupational history as risk indicators for low back trouble in the general population." Spine 11:720-5
22) Balague F, Nordin M, et al. "recovery from severe sciatica." Spine 1999; 24:2516-24.
23) Saal JA, et al. “The natural history of lumbar intervertebral disc extrusions treated nonoperatively.” Spine – 1990; 15:683-686
25) Mixter WJ, Barr JS. “Rupture of the intervertebral disc with involvement of the spinal canal.” N Engl J Med 1934; 211:210-5
29) van Akkerveeken PF. “Pain patterns and diagnostic blocks.” In: Wiesel SW, Weinstein JN, Herkowitz H, eds. The Lumbar Spine. Philadelphia: W.B. Saunders Company, 1996:105-22.
30) Foerster O: "Zur kenntniss der spinalen segmentinnervation der muskelin." Neurol Zbl 32:1202-1214, 1913
31) Uihlein A, et al. "neurologic changes, surgical treatment, and post operation evaluation. Symposium: Low back and sciatic pain." J Bone Joint Surg 50A:1, 1968
32) Bolk L. "Die Segmentaldifferenzigrung des menschlichen Rumpfes und seiner Extremitaten." morphol Jahrb 1898 - 1899; 25:465-543; 26:91-211; 27:630-711; 28:105-46
33) Konstantinou K, Dunn KM. "sciatica: review of epidemiological studies and prevalence estimates." Spine 2008; 33:2464-2472.
40) Jensen MC, et al. “MRI imaging of the lumbar spine in people without back pain.” N Engl J Med – 1994; 331:369-373
41) Boden SD et al. “Abnormal magnetic resonance scans of the lumbar spine in asymptomatic subjects: A prospective investigation.” J Bone Joint Surg Am 1990; 72A:403-408
42) Weishaupt D et al. “MRI of the lumbar spine: Prevalence of intervertebral disc extrusion and sequestration, nerve root compression and plate abnormalities, and osteoarthritis of the fact joints in Asymptomatic Volunteers.” Radiology – 1998; 209:661-666
43) Boos N, et al. “1995 Volvo Award in clinical science: The diagnostic accuracy of MRI, work perception, and psychosocial factors in identifying symptomatic disc herniations.” Spine – 1995; 20:2613-2625
44) Powell MC, et al. “Prevalence of lumbar disc degeneration observed by magnetic resonance in symptomless women.” Lancer – 1986; 2:1366-7
45) Boos N, et al. “Natural history of individuals with asymptomatic disc abnormalities in MRI: Predictors of low back pain-related medical consultation and work incapacity.” Spine 2000; 25:1484
46) Borenstein G, et al. “The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic individuals: A 7-year follow-up study. J Bone Joint [am] 2001; 83:320-34
47) Wiesel SW, et al. “A study of computer-associated tomography: I. The incidence of positive CAT scans in asymptomatic group of patients.” Spine 1984;9:549-51
48) Leonardi M, Pirrman CW, Boos N (2006). "injection studies in spine disorders." Clin Orthop Relat Res 443:168-82.
49) Vroomen PC, de Krom MC, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002; 52:119-123.
50) Nygaard OP, Kloster R, Solberg T. Duration of leg pain as a predictor of outcome after surgery for lumbar disc herniation: a prospective cohort study with one-year follow-up. J Neurosurg. 2000; 92 (2 supppl): 131-134.
51) Jannson KA, et al. health-related quality of life in patients before and after surgery for a herniated lumbar disc. J Bone Joint Surg Br. 2005; 87:959-964.
52) Rydevik B, Brown MD, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine 1984; 9:7-15.
53) McCulloch JA (1996) Focus issure on lumbar disc herniation: macro-and microdiscectomy." Spine 21:45S-56S.
54) Garfiin SR, Rydevik BL, Brown RA. Compressive neuropathy of spinal nerve roots: a mechanical or biological problem? Spine 1991;16:162-166.
55) Sunderland S. Nerves and nerve injuries. Second edition. Edinburgh, London and New York, Churchill Livingstone, 1978.
56) Hasue M. Pain and the Nerve Root: An interdisciplinary approach. Spine 1993;18:2053-2058.
57) Geiss A Larsson K, Rydevik B et al. Autoimmune properties of nucleus pulposus. Spine 2007; 32:168-173.
58) Habtemariam A, Virri J, Gronblad M,et al. Inflammatory cells in full-thickness annulus injury in pigs: An experimental disc herniation animal model. Spine 1998; 23:524-529.
59) Abe Y, Akeda K, An HS,et al. Pro-inflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine 2007; 32:635-642.
60) Park JO et al. Inflammatory cytokines induce fibrosis and ossification of human ligamentum flavum cells. J Spinal Disord Tech 2013; 26:E6-12.
61) Tachihara H, Kikuchi S, Konno s, Sekiquchi M. Does facet joint inflammation induced radiculopathy? An investigation using a rat model of lumbar facet joint inflammation. Spine 2007;15:406-412.
62) Comer CM, et al. Assessment and management of neurogenic claudication associated with lumbar spinal stenosis ina UK primary care musculoskeletal service: a survey of current practice among physiotherapists. BMC Musculoskelet Disord 2009;10:121.
63) Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med 1934; 211:210-225.
64) Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations of the lumbar spine using local anesthesia. Orthop Clinics North Am 1991;22:181-187.
65) Smyth MJ, Wright V. Sciatica and the intervertebral disc: an experimental study. J Bone Joint Surg Am 1958;40-A:1401-1418.
66) Ooi Y, et al. Myeloscopic study on lumbar spinal canal stenosis with special reference to intermittent claudication. Spine 1990;15:544-549.
67) Coventry MB, et al. “The intervertebral disc: Its microscopic anatomy and pathology: Part II. Changes in the intervertebral disc concomitant with age.” J Bone Joint Surg 27A:233-252
68) Mixter WJ, Barr JS. “rupture of the intervertebral disc with involvement of the spinal canal.” N Engl J Med 211:210-214 1934
69) Vernon-Roberts B, Pirie CJ. “Degenerative changes in the intervertebral disc of the lumbar spine and their sequealae.” Rheum Rehab 16:13-21 1977
70) Karppinen J, et al. “Severity of Symptoms and Signs in Relation to Magnetic Resonance Imaging Findings Among Sciatic Patients.” Spine 2001; 26:E149-E154
71) Taylor CS, Coxon AJ, Watson PC, Greenough CG. Do L5 and S1 nerve root compressions produce radicular pain in the dermatomal pattern? Spine 2013; 38:995-998.
72) Tullberg T, Svanborg E, Isacsson J, Grane P. A preoperative and postoperative study of the accuracy and value of electro diagnosis in patients with lumbosacral disc herniation. Spine 1993;18:837-842.
77) Saal & Saal, "Nonoperative Treatment of Herniated Lumbar IVD with Radiculopathy: An outcome Study." Spine 1989; 14(4):431-437.
78) Henrik Weber , '1982 Volvo Award in Clinical Science' "Lumbar Disc Herniation: A controlled, Prospective Study with Ten Years of Observation." Spine 1983; 8(2):131-140.
79) van der Windt DA, Simons E, Riphagen II, et al. Physical examination for lumbar radiculopathy due to disc herniation in patients with low-back pain. C et al.och et al.rane Database Syst Rev 2010;17:CD007431.
80) Nakamura SI, Takahashi K, Takahashi Y, et al. "Origin of nerves supplying the posterior portion of lumbar Intervertebral discs in rats." Spine 1996; 21:917-924.
81) Nakamura SI, Takahashi K, Takahashi Y, et al. "The afferent pathways of discogenic low-back pain. Evaluation of L2 spinal nerve infiltration." J Bone Jont Surg. 1996;78:606-612
82) Mahn F, Hullemann P, Gockel U,et al. Sensory symptom profiles and comorbidities in painful radiculopathy. PLoS ONE 2011;6:1-7.
83) Ikawa M, Atsuta Y Tsunekawa H. Ectopic firing due to artificial venous stasis in rat lumbar spine canal stenosis model: a possible pathogenesis of neurogenic intermittent claudication. Spine 2005;30:2393-2397.
84) Porter RW. Spinal stenosis and neurogenic claudication. Spine 1996; 21:2046-2052.
85) Hancock MJ, Koes B, Ostelo R, Peul W. Diagnostic accuracy of the clinical examination in identifying the level of herniation in patients with sciatica. Spine 2011; 36:E712-E719.
86) Kortelainen P, Puranen J, Koivisto E, et al. Symptoms and signs of sciatica and their relation to the localization of the lumbar disc herniation. Spine 1985; 10:88-92.
87) Al Nezari NH, et al. Neurological examination of the peripheral nervous system to diagnose lumbar spinal disc herniation with suspected radiculopathy: a systematic review and meta-analysis. Spine J 2013;13:657-674.
88) Savage NJ, et al. The relationship between history and physical examination findings and the outcome of electrodiagnostic testing in patients with sciatica referred for physical therapy. J Orthop Sports Phys Ther 2014; ahead of print.
89) Peng B, Wu W, Li Z, et al. Chemical radiculitis. Pain 2007;127:11-16.
90) Kim KY, et al. Magnetic resonance imaging in the evaluation of lumbar herniated intervertebral disc. Int Orthop 1993;17:241-244.
91) Inal EE, et al. Comparison of clinical and electrophysiological findings in patients with suspected radiculopathies. JBack Musculoskeletal Rehabil 2013; 26:169-173.
92) Tong HC. Specificity of needle electromyography for lumbar radiculopathy and 55- to 79-yr-old subjects with low back pain and sciatica without stenosis. Am J Phys Med Rehabil 2011; 90:233-238; quiz 239-42.
95) Cooper RG, Freemont AJ, “TNF-a blockade for herniated intervertebral disc-induced sciatica: a way forward at last?” Rheumatology 2004; 43:119-121.
96) Olmarker K, Rydevik B, et al. “Autologous nucleus pulposus induced's neurophysiologic and histologic changes in porcine cauda equina nerve roots.” Spine 1993; 18:1425-32
97) Olmarker K, et al. “Inflammatory properties of nucleus pulposus.” Spine 1995; 20:665-9
98) kayama S, Olmarker K, et al. “Incision of the annulus fibrosus induces nerve root morphological, vascular and functional changes: an experimental study.” Spine 1996; 21:2539-43
99) Olmarker K, Brisby H, et al. “The effects of normal, frozen and hyaluronidase-digested nucleus pulposus on nerve root structure and function.” Spine 1997; 22:471-5.
100) Nygaard OP, Mellgren SI. The function of sensory nerve fibers in lumbar radiculopathy: use of qualitative sensory testing in the exploration of different populations of nerve fibers and dermatomes. Spine 1998; 23:348-353.
126) Balague F, et al. "Recovery of Severe Sciatica." 1999; 24(23):2516-2524
140) Karppinen J, et al. “Tumor necrosis factor-alpha Monoclonal Antibody, Infliximab, Used to manage severe sciatica.” Spine; 28(8):750-753
141) Igarahi T, et al “Volvo Award Winner 2000” Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology: Molecular, histologic, and behavioral comparisons in rats.” Spine 2000; 25:2975-80
144) Blumenauer B, et al. “Infliximab for the treatment of rheumatoid arthritis.” Cochrane Database Syst Rev 2002; 3:CD003785
145) Maini R, et al. “Infliximab (Chimeric Antitumor necrosis factor-alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A randomized phase III trial. ATTRACT Study Group. Lancet 1999; 354:1932-9.
146) Chaudhari U, et al. “Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: A random trail. Lancet 2001; 357:1842-7
147) Braun J, et al. “Treatment of active ankylosing spondylitis with infliximab: A randomized controlled multicentre trial.” Lancet 2002; 359:1187-93
148) Hanauer SB, et al. “maintenance infliximab for Crohn’s disease: The ACCENT I randomized trial. Lancet 2002;359:1541-9
153) Olmarker K, Rydevik B, et al. “Autologous nucleus pulposus induces neurophysiologic and histologic changes on porcine cauda equina nerve roots.” Spine 1993 18:1425-1432
155) Olmarker K, Rydevik B, et al. “ultrastructural changes in spinal nerve roots induced by Autologous nucleus pulposus.” Spine 1996; 21:411-414
181) Heliovaara M, et al. “Lumbar disc syndrome in Finland.” J Epidemiol Commun Health 1987; 41:251-258
182) Manninen P, et al. “Incidence and risk factors of low-back pain in middle-aged farmers.” Occup med 1995; 45:141-146
183) Svensson HO, Andersson GBL. “Low back pain in forty to forty-seven year old men: I Frequency of occurrence and impact on medical services.” Scand J Rehabil Med. 1982; 14:47-53
184) Svensson HO, Andersson GBL. “Low back pain in forty to forty-seven year old men: work history and work environment factors.”Spine 1983 8:272-276
185) Svensson HO, Andersson GBL. “The relationship of low back pain, work history, work environment, and stress: A retrospective cross-sectional study of 38- to 64-year old women.” Spine 1989; 14:517-522
186) Svensson HO, Andersson GBL. “A retrospective study of low back pain in 38- to 64 year old women: frequency and occurrence and impact on medical services.” Spine 1988; 13:548-522
[190] Boos N, et al. “1995 Volvo Award in Clinical Sciences: The Diagnostic Accuracy of Magnetic Resonance Imaging, Work Perception, and Psychosocial Factors in Identifying Symptomatic Disc Herniations.” Spine 1995; 20:2613-2625
191) Spencer DL. Mechanisms of nerve root compression due to a herniated disc. In: Weinstein JN, Wiesel SW, International Society for the Study of the Lumbar Spine, eds. The Lumbar Spine. Philadelphia: WB Saunders, 1990:141-5.
192) Kelsey JL. An epidemiological study of the relationship between occupations and acute herniated lumbar intervertebral discs. Int J Epidemiol 1975;4:197-205.
193) Kelsey JL, Githens PB, White AA. An epidemiologic study of lifting and twisting on the job and risk for acute prolapsed lumbar intervertebral discs. J Orthop Res 1984;2:61-6.
194) Mundt DJ, Kelsey JL, Golden AL, et al. An epidemiologic study of non-occupational lifting as a risk factor for herniated lumbar intervertebral disc. Spine 1993;18:595-602
195) Videman T, Nurminen M, Troup JD. 1990 Volvo Award in Clinical Sciences. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation, and physical loading. Spine 1990;15:728-40.
257) Marshall LL, et al. “Chemical irritation of nerve root in disc prolapse.” Lancet 1973; 2:320
258) Marshall LL, et al. “Chemical Radiculitis: A clinical, physiological and immunological study. Clin Orthop 129:61-67, 1977
281) Monteiro A et al. “lateral decompression of a pathological disc in the treatment of lumbar pain & sciatica.” Clin Orthop 1989; 56-63
282) Schreiber A, et al. “Does percutaneous nucleotomy with discoscopy replace conventional discectomy? Eight years of experience and results in treatment of herniated lumbar disc.” Clin Orthop 1989; 35-42
[290] Kortelainen P, et al. “Symptoms and signs of sciatic and their relation to the location of the lumbar disc herniation.” Spine – 1985; 10:88-92
291) Hirsch C, Nachemson A. “The reliability of lumbar disc surgery.” Clin Orthop 1963; 29:189-194
301) Dauch WA, et al. "predictors of treatment success after microsurgical operation of lumbar intervertebral disc displacements" Zentralbl Neurochir - 1994; 55:144-155
302) Dvorak J, et al. "The outcome of surgery for lumbar disc herniation." Spine -1988; 13:1418-22
303) Hurme M and Alaranta H "Factors predicting the result of surgery for lumbar intervertebral disc herniation. Spine - 1987; 12:933-938
304) Postacchini F, "Management of herniation of the lumbar disc." J Bone Joint Surg - 1999; 81- B :567 -576
305) Rothoerl RD, et al. "When should conservative treatment for lumbar disc herniation be ceased and surgery considered?" Neurosurg Rev - 2002; 25:162-165
306) Davis RA, et al. “A long-term outcome analysis of 984 surgically treated herniated lumbar discs.” J Neurosurg – 1994; 80:415-21
307) Hakelius A, “Prognosis in sciatica: a clinical follow-up of surgical and non-surgical treatment.” Acta Orthop Scand 1970; Suppl 129
308) Salenius P, “Results of operative treatment of lumbar disc herniation. Acta Orthop Scand 1977; 48:630-4
309) Spengler DM, et al. “Elective discectomy for herniation of lumbar disc: additional experience with an objective method.” J Bone Joint Surg – 1990; 72A:230-237
550) Olmarker K, Larsson K. “Tumor necrosis factor alpha, and nucleus-pulposus-induced nerve root injury.” Spine 1998; 23:2538-2544
551) Kayama S, Olmarker K, et al. “Incision of the anulus fibrosus induces nerve root morphologic, vascular, and functional changes: An experimental study.” Spine 1996;21:2539-43
552) Olmarker K, Brisby H, Rydevik B, et al. “The effects of normal, frozen, and hyaluronidase-digested nucleus pulposus on nerve root structure and function.” Spine 1997; 22:471-5discussion 476
553) Olmarker K, Rydevik B, et al. “Effects of methylprednisolone on nucleus pulposus-induced nerve root injury.” Spine 1994; 19:1803-8
554) Olmarker K, Rydevik B, et al. “Ultrastructural changes in spinal nerve roots induced by autologous nucleus pulposus.” Spine 1996; 21:411-4
555) Olmarker K et al. “Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots [see comments] Spine 1993; 18:1425-32
556) Byrod G, Reydevik B, Olmarker K, et al. “Acute increase in endoneural vascular permeability induced by epidural application of nucleus pulposus on spinal nerve roots. Manuscript [550]
557) Otani K Olmarker K, et al. “Nucleus pulposus-induced increased in vascular permeability in the nerve root. Manuscript [550]
558) Olmarker et al. “Inflammatogenic properties of nucleus pulposus.” Spine 1995;20:665-9
619) Ohnmeiss DD, et al. "Degree of disc disruption and lower extremity pain" Spine 1997; 22(14):1600-1605
620) Ohnmeiss DD, et al. "Relation between pain location and disc pathology: a study of pain drawings and CT/discography." Clin J Pain 1999 ;15:210-7.
621) Milette PC, et al. “Radiating Pain to the Lower Extremities Caused by Lumbar Disk Rupture without Spinal Nerve Root Involvement.” AJNR Am J Neuroradiol 1995; 16:1605-1613
788) Marshall LL, et al. “Chemical irritation of nerve root in disc prolapse.” Lancet 1973; 2:320
789) Marshall LL, et al. “Chemical Radiculitis: A clinical, physiological and immunological study. Clin Orthop 129:61-67, 1977
790) Olmarker K, Rydevik B, et al. “Autologous nucleus pulposus induced neurophysiologic and histologic changes in porcine cauda equina nerve roots.” Spine 1993; 18:1425-32
791) Olmarker K, Rydevik B, et al. “ Ultrastructural changes in spinal nerve roots induced by autologous nucleus pulposus.” Spine 1996; 21:411-4
792) Olmarker K, et al. “Inflammatory properties of nucleus pulposus.” Spine 1995; 20:665-9
793) kayama S, Olmarker K, et al. “Incision of the annulus fibrosus induces nerve root morphological, vascular and functional changes: an experimental study.” Spine 1996; 21:2539-43
794) Olmarker K, Brisby H, et al. “The effects of normal, frozen and hyaluronidase-digested nucleus pulposus on nerve root structure and function.” Spine 1997; 22:471-5
795) Olmarker K, Larsson K. “Tumor necrosis factor alpha, and nucleus-pulposus-induced nerve root injury.” Spine 1998; 23:2538-2544
800) Brisby H, Rydevik B, et al. " Glycosphingolipid antibodies in serum in patients with sciatica." Spine 2002 ;27(4):380-6.
801) Chacur M, et al. "A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats." Pain 2001 ;94(3):231-4
900) Korhonen T, Karppinen J, et al. 'Efficacy of infliximab for disc herniation-induced sciatica: one-year follow-up.' Spine. 2004 Oct 1;29(19):2115-9
© Copyright 2002 – 2005 by Dr. Douglas M. Gillard DC - All rights reserved