Spondylolisthesis & Spondylolysis |
 |
General Information | Classification Systems |Types of Spondylolisthesis | Isthmic Spondylolisthesis | Spondylolysis | Degenerative Spondylolisthesis | Last Updated 3/11/17 |
If you really want to get into this subject, check out my classroom lectures (as posted on YouTube) on this topic:
Spondylolysis/spondylolisthesis and then, if you dare, take the "quiz" which covers spondylolisthesis and much more! The Spondylolisthesis and Other Condition Quiz.
Definition: a spondylolisthesis occurs when the top vertebra of a motion segment (remember a motion segment is made up of two adjacent vertebrae and the disc in between them) slides (slips, or translates) forward in relationship to the bottom vertebra. Such movement is technically called sagittal anterior translation.
 Anatomy Review: Please recall from the anatomy page that vertebrae (especially the bottom two, L4 and L5) have a natural tendency to slide forward, especially during forward flexion of the trunk. This natural slip tendency is stopped by having an intact posterior arch, which consists of pedicles, articular pillars, pars interarticulari, and laminae, as well as intact zygapophyseal joints (a.k.a., facet joints or Z joints).
Anatomy Review: Please recall from the anatomy page that vertebrae (especially the bottom two, L4 and L5) have a natural tendency to slide forward, especially during forward flexion of the trunk. This natural slip tendency is stopped by having an intact posterior arch, which consists of pedicles, articular pillars, pars interarticulari, and laminae, as well as intact zygapophyseal joints (a.k.a., facet joints or Z joints).
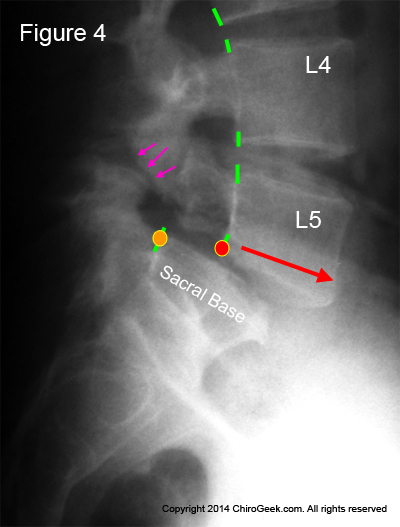
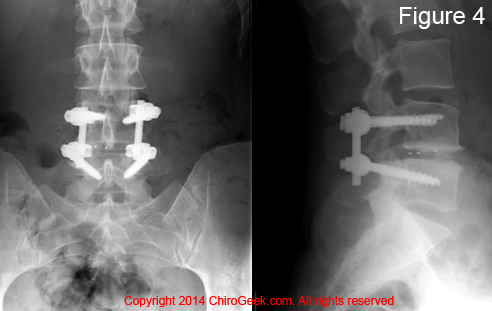
Figure #4: This is a run-of-the-mill isthmic spondylolisthesis (Type II-A) at L5/S1. Note that the vertebral body of L5 has slid approximately 50% forward in relation to the sacral base (body of S1). If you look closely (pink arrows), you can see the actual pars interarticularis fracture.
Why does it occur? Any failure of any portion of the posterior arch and/or facet joints may ultimately result in the slip of spondylolisthesis. This typically occurs secondary to either "stress fractures" in the pars interarticularis (this is called an Type II-Type A isthmic spondylolisthesis) and/or or failure of the facet joints (this is call a Type III degenerative spondylolisthesis).
Can they be a source of chronic back and/or leg pain? The short answer is, "yes." Although not all spondylolistheses are symptomatic, a significant percentage of them are and can result in chronic lower back pain, as well as chronic radicular pain (radiating lower extremity pain). Specifically, epidemiological studies have shown that a little over 50% of these are completely asymptomatic. This 50% rule, however, does not hold for young athletes, for they are at much higher risk for the discovered spondylolytic defect being symptomatic.
The first thing we need to classify is the magnitude of the slip. The most common system use today was developed by Meyerding way back in 1931. [Meyerding HW. Spondylolisthesis. Bone Joint Surg 1931; 13:39-48] Let's talk about it:
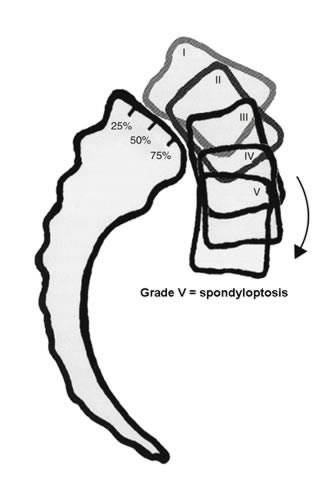 It's a pretty simple concept, which goes like this:
It's a pretty simple concept, which goes like this:
Grade I = if the top member of the motion segment has slipped (moved forward) between 0 and 25%, then you are said to have a grade 1 spondylolisthesis.
Grade II = if the slip is between 25% and 50%, then you are said to have a grade II spondylolisthesis.
Grade III = if the slip is between 50% and 75%, then you are said to have a grade III spondylolisthesis.
Grade IV = if the slip is between 75% and 100%, then you are said to have a grade IV spondylolisthesis.
*Grade V (a.k.a., spondyloptosis) = if the slip exceeds 100%, then you are said to have a grade V spondylolisthesis or, more commonly, a spondyloptosis.
*not part of the original Meyerding system.
Here's a few more definitions you should become familiar with:
Low-grade spondylolisthesis: if the slip is less than 50%, then you are said to have a low grade spondylolisthesis. [Meyerding (1932)] Degenerative spondylolisthesis is always of this type, and so is isthmic spondylolisthesis for the most part.
High-grade spondylolisthesis: if the slip is greater than 50%, then you are said to have a high grade spondylolisthesis (which is not a good thing!) Type I dysplastic spondylolisthesis is typically the culprit here.
The second thing we need to classify is the types of spondylolistheses.
Although there are several classification systems, the one most commonly used and widely accepted was proposed by Wilste back in 1976 [Clin Orthop Relat Res 1996; 117:23-9.] Let's talk about the six different types of spondylolisthesis.
Dysplastic Spondylolisthesis (Type I):
Isthmic Spondylolisthesis (Type II):
Degenerative Spondylolisthesis (Type III)
Traumatic Spondylolisthesis (Type IV)
Pathological Spondylolisthesis (Type V)
Iatrogenic Spondylolisthesis (Called Type II-C in Some Systems)
It is beyond the scope of this webpage to get into all six of these. However, I will cover in depth the two most common types of spondylolisthesis that clinicians encounter on a daily basis: the isthmic spondylolisthesis and a degenerative spondylolisthesis. Let's talk about them!
Natural History | Why Does It Slip? | Pain Generators | Treatment Options | Surgical Options |
An isthmic spondylolisthesis typically results from a stress fracture of the pars interarticularis (the thinnest part of the posterior arch) which then fails to stop the natural anterior translation of the affected vertebra, upon its motion segment mate below.
The prevalence (how many people have this in the population) of isthmic spondylolisthesis in North America is approximately 4% to 7% in Caucasians and African-Americans. In certain populations, however, it is very high. For example, in Inuits, it has been demonstrated at 45%! [Rowe (1953), Whitesides et al (2005)]
Fun Facts: Unlike degenerative spondylolisthesis which is commonly seen at L4, isthmic spondylolisthesis typically affects the L5 vertebra.
There are three different "flavors" (types) of isthmic spondylolisthesis:
 Type II-A (pars stress fracture) = this is your run-of-the-mill pars interarticularis stress fracture which is typically occurs in the first or second decade of life, especially in young athletes.
Type II-A (pars stress fracture) = this is your run-of-the-mill pars interarticularis stress fracture which is typically occurs in the first or second decade of life, especially in young athletes.
Type II-B (pars elongation) = the slip has been caused by a series of pars fractures, and then healing. With each fracture-heal cycle, the pars interarticularis gets longer and longer and pushes the vertebral body farther and farther forward.
Type II-C (acute pars fracture) = this is actually quite rare, but occurs when a single specific incident fractures the pars interarticularis. Some newer authors have eliminated this subcategory and created a new one called an Iatrogenic Spondylolisthesis.
For you clinicians out there, the slip not only can cause chronic lower back pain, but can also compress one or both of the exiting nerve roots in the lateral recess or neuroforamen, resulting in often severe radicular pain. This condition does not typically cause the central stenosis seen in degenerative spondylolisthesis.
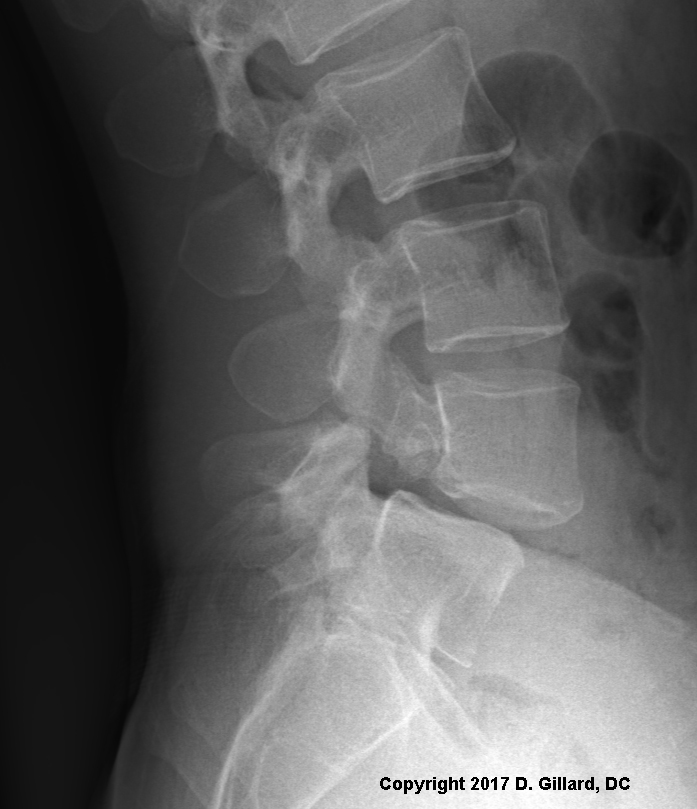
Figure Left: This is a Sagittal radiograph of a run-of-the-mill Isthmic Spondylolisthesis at L4/5 which would be classified as a Grade I Isthmic Spondylolisthesis. Without knowing the patient history, you can't say for sure that it is type II-A vs. type-II-C. If you look closely at the radiographic image, you can see the fractures through both pars interarticulari. Can you see them? If not, click here.
The Type 2-B (pars elongation) spondylolistheses are pretty rare to catch on radiograph. Why? Please recall these are the ones where the pars interarticularis gets longer and longer each time the pars fractures and then heals. This fracture-heal-fracture-heal cycle results in a pars interarticularis that becomes very long and sometimes slender, a finding that is called in some circles the "greyhound sign."
 Why are these spondylolistheses rare to actually catch in your office?
Why are these spondylolistheses rare to actually catch in your office?
Because most of the time, the patient will have fractured the pars again which has caused the flareup of pain that has brought him or her into your office; therefore, it will appear as a Type II-A on your radiograph.
Let's take a look at one, which was from a recent client of mine [don't forget, I offer a coaching service to help you make decisions regarding the treatment of these conditions] of mine, with permission.
On the Image Left, you can clearly see that L4 has slipped about 25% on L5 (i.e., a grade 1 spondylolisthesis), but can you see the cause of the slip? If you can't, click here.
Question Answer:
Clearly, the right pars interarticularis (the left one exactly the same) on the CT sagittal is way too long (Greyhound sign) and is also too thin (dysplastic).
Therefore, we can easily classic this defect as a Type II-B isthmic spondylolisthesis.
The 35-year-old female has had low back pain for 20 years and was a top-level gymnast who had multiple pars interarticularis fractures during her career.
The Natural History of Isthmic Spondylolisthesis: the Fredrickson & Beutler study
Although we shall talk more specifically about spondylolysis further below, at this point I need to mention it because some of you may be wondering just what has caused the isthmic spondylolisthesis.
Definition: a "spondylolysis" is a fracture of the posterior arch, which almost always occurs at its weakest point (the pars interarticularis), but this fracture has not yet allowed in the slip to occur.
So a fracture of the pars interarticularis may at first not allow slip (this would be called a spondylolysis) or it may immediately allow slip (this would be called a spondylolisthesis).
Let's talk a bit about a classic and fascinating study, first published by Fredrickson et al Back in 1984 [Fredrickson BE, et al. The Natural History of Spondylolysis and Spondylolisthesis. J Bone and Joint Surg AM 1984; 66:699-707.] This original study was carried on for 45 years and the results were published by Beutler & Fredrickson in 2003. [Beutler WJ, Fredrickson BE, et al. The Natural History of Spondylolysis and Spondylolisthesis: 45-Year Follow-Up Evaluation. Spine 2003; 28:1027-1035.
The research team performed radiographs (took x-rays) of 500 consecutive first-graders (six years old) looking for spondylolysis and/or spondylolisthesis.
Results indicated that 4% of these young students had either a spondylolysis or a spondylolisthesis which was rarely symptomatic. In other words, most of these were just incidental findings in did not, at least yet, correlate with low back pain or leg pain.
At age 25, the spondylolytic children (now adults) were reevaluated.
Surprisingly, the magnitude of slip had worsened in 81% of them!
At the 45 year follow-up, it was discovered that 52% of those affected originally with spondylolysis or spondylolisthesis had suffered at least one major episode of low back pain and 10% had succumbed to spine surgery! Just to put that in perspective, normally only 1% to 2% of the population undergoes spine surgery, not 10%!
Of the participants with unilateral spondylolysis (0.8%), 37.5% of them had no radiographic evidence of the spondylolysis by age 31.
In a separate arm of the study, 500 newborn babies were x-rayed looking for signs of spondylolysis or spondylolisthesis. Not a single one was found!
RISK FACTORS:
It has been demonstrated that certain sports increase the likelihood of a child or young adult developing spondylolysis or isthmic spondylolisthesis.
Specifically, sports such as gymnastics and rowing approximately doubled the chance [51], and the throwing events of track and field (hammer, discuss, shot put and javelin) increase the likelihood by a factor of eight! [51] Other high risk sports include American football (especially offense of linemen), diving, wrestling, and swimming.
Since there are only two osseous structures that hold the vertebral body in its normal position within the spine, i.e., the posterior arch and the facet joints, the logical answer to the question, "what causes the slip?" would be a problem with one or both of these structures. And that is a correct conclusion. (*On rare occasions, people can develop a congenital lengthening of the pedicles, which in turn results in spondylolisthesis. This type of spondylolisthesis is called a dysplastic spondylolisthesis and is beyond the scope of this page.)
In isthmic spondylolisthesis, however, the slip is caused from a fracture of the pars and has nothing to do with the facet joints (see degenerative spondylolisthesis). When such a fracture occurs, the posterior arch then becomes incapable of stopping the natural tendency of the vertebral body to slide forward on the vertebral body below.
 Natural Anterior Translation
Natural Anterior TranslationOkay, at this point I can hear you say, "But why does the vertebral body have a natural tendency to slide forward?" and "what is natural anterior translation?". Let me elaborate:
Just like the tendency of a ball to roll down a hill, the bottom three vertebrae, which are where the vast majority of isthmic spondylolistheses occurs, [20,44,45] want to roll (or really slide) down the hill that they are sitting on—namely the vertebrae below.
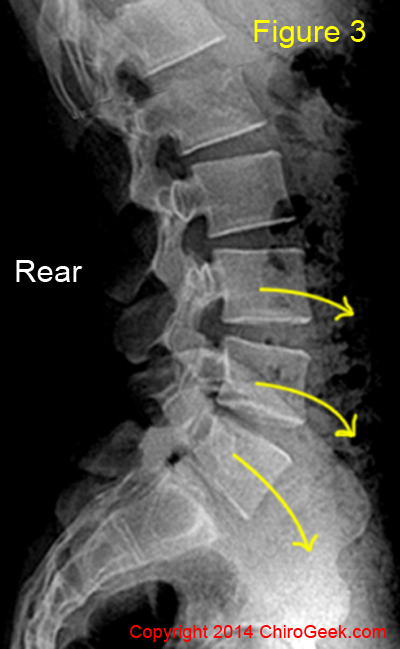
Remember from the anatomy page that the normal lumbar spine has a lordotic curve as viewed from the side (figure 3). Such a curve results in the bottom three vertebrae being angled (cocked) forward to such a degree that an incline developes, which in turn makes them ready to slide on one another under the influence of gravity. (*Biomechanically speaking, there is more to this story, but that is certainly good enough for our scope.)
In figure 3, the yellow curved arrows are an attempt to represent the tendency of the lower three lumbar vertebrae to translate (slide) down the vertebra below, a tendency dependent upon gravity.
In addition to the role of gravity, biomechanical studies have demonstrated that forward flexion of the lumbar spine (bending forward at the waist) also forces the vertebrae to anterior translate, which is normally prevented by the facet joints and the pars of the posterior arch.
*Warning: figuring out why you have pain and where the pain is coming from is very complicated. It takes years of study and practice to become a good spine doctor, and the forthcoming information is certainly not enough to enable you to make an accurate self-diagnosis. I do, however, offer a Coaching Service which will allow you and me to dig much deeper into what has went wrong with your spine, as well as formulate a plan on what to do about it.
There are three pain-patterns that are typically related to isthmic spondylolisthesis: mechanical low back pain, radicular pain, and neurogenic claudication. [60] Let's talk about them each.
Mechanical low back pain is a syndrome that occurs when the patient's low back pain is set-off by movements at the waist (flexion, extension, lateral bending, and rotation) as performed during the activities of daily living or greater. In other words, the patient develops back pain during activities such as getting dressed, going to the restroom, showering, preparing meals, housecleaning, doing yard work, and/or running errands. After resting in a recliner or in bed, however, the pain eventually settles down.
Where does the pain come from? That my friends is the $64,000 question and will be different for every person who is affected.
The typical cause is thought to be from micro-motion within the fractured pars interarticularis, which typically cannot heal on its own because of a poor natural blood supply. The body does, however, try to heal the fracture by growing new blood vessels into the fracture site which are used to form a tangled mess of collagen in the defect (scar tissue). Unfortunately, where new blood vessels go, pain-carrying nerve fibers will follow which results in the pars defect becoming filled with pain-producing nerve fiber, which in turn is triggered by simple movement at the waist under the influence of gravity. In simple terms, the pars fracture hurts when you move.
*Low back pain can also come from an irritation of the nociceptive fibers within the disc, vertebral endplates, and/or facet joints, secondary to biomechanical changes within the motion segment which inevitably occur after the development of spondylolysis and/or isthmic spondylolisthesis. Therefore, you may have more than one pain generator with isthmic spondylolisthesis.
Radicular pain (a.k.a., lower extremity pain, leg pain, or sciatica) classically presents as a unilateral terrible burning, electric-like, and/or throbbing type of pain that runs from buttock, down the lower extremity and often into the foot. It initially may mirror the dermatome of the affected nerve, but can also become non-dermatomal in patients with chronic (long-standing) pain.
What causes the radicular pain? There are two causes: #1) the fracture site it self can produce a thickening which can irritate the exiting nerve root as it rounds the pedicle and passes near the fracture site. This "thickening" is called the beak of the spondylolisthesis/spondylolysis. #2) if there is a slip, then the superior articular process can slide forward and irritate the exiting nerve root in the lateral recess and/or proximal neuroforamen.
(If I just lost you, then you have not studied the disc anatomy page hard enough and might want to consider revisiting that page.)
Neurogenic claudication (a.k.a., neurogenic intermittent claudication) is one of the more severe complications of isthmic spondylolisthesis (or degenerative spondylolisthesis for that matter) and occurs after prolonged standing or walking. Specifically, the person will experience a progressive increase of lower extremity pain, cramping, and weakness following standing or walking, which is dramatically relieved by sitting down to rest or bending forward at the waist—like when you push a shopping cart {this is called "shopping cart sign"}). [63] this neurogenic intermittent claudication, unlike in central stenosis caused by degenerative spondylolisthesis, typically ONLY AFFECTS ONE LOWER EXTREMITY.
By this point, I bet many of you are wondering about the available treatment options for isthmic spondylolisthesis and spondylolysis. Therefore, let's talk about them.
Warning: this is only for educational purposes. I would be happy to discuss this in more detail with you personally during a GoToMeeting coaching session, which you can read all about here.
I shall not dive to deeply into the multifaceted subject of nonoperative care (conservative care); however, I will say that the lions' share of symptomatic isthmic spondylolisthesis and spondylolysis patients respond quite nicely to treatment interventions such as medication, bracing, physical therapy, low-force chiropractic care (never let your chiropractor perform a grade 5 spinal manipulation!), acupuncture, and home exercise. Injective treatments, such as epidural steroid injections (for lower extremity pain), and facet / pars injections (for low back pain) may also be effective if the other non-operative treatments fail.
YOU ALWAYS WANT TO TRY ALL FORMS OF CONSERVATIVE CARE BEFORE SUCCUMBING TO SURGERY!
For patients of whom were refractory to non-operative care, the end of the road treatment is spine surgery.
What are the absolute indications? According to a highly respected spine surgeon, author, and researcher, there are only four indications for isthmic spondylolisthesis or spondylolysis fusion: [25]
If you are a candidate for surgery, then the $64,000 question becomes what type of surgery do I get? That is a very complicated question, the answer of which will vary depending on your unique circumstances. However, there are some general guidelines which I can offer. I should also warn you, that if you haven't already done so, you should visit my FUSION PAGE, so you understand what I'm talking about when I say things like, "TLIF." I will assume you understand the basics of fusion surgery.
What is the rate of surgery for isthmic spondylolisthesis? In 2016, Thirukumaran et al published the results of their study on this very question. [74] After analyzing over 47,000 surgeries, they discovered the annual rate of fusion surgery in 2011 for the treatment of isthmic spondylolisthesis was 122.6 surgeries per million adults. Because there are approximately 242 million adults in the United States, this translates into approximately 30,000 surgeries per year, in the United States alone!
Furthermore, the researchers discovered the rate of surgery is dramatically increasing. Specifically, the annual rate of fusion surgery for isthmic spondylolisthesis increased 4.33 times between 1998 and 2011! [74]
 What type of surgery is becoming popular for isthmic spondylolisthesis?
What type of surgery is becoming popular for isthmic spondylolisthesis?
Again, Thirukumaran et al demonstrated that the posterior procedures (transforaminal lumbar interbody fusion and posterior lateral interbody fusion) are becoming far more popular than anterior lumbar interbody fusion and/or 360° fusion (PLIF + PLF). How much more popular? Since 1998, there has been a 330% increase in the use of the posterior procedures; the anterior procedure has really fallen out a favor.
Why has anterior lumbar interbody fusion, with or without posterolateral fusion, fallen out a favor?
First of all, to this very day (1/3/17) there is still no convincing evidence that anterior lumbar interbody fusion or ALIF + PLF (i.e., the 360° fusion) affords better clinical outcomes than the posterior procedures. [77-80] Why could that be? It may have something to do with the known higher rate of revision surgery which ALIF + PLF (a.k.a., 360° fusion) have. Specifically, Thirukumaran (2016) discovered in their study of over 47,000 surgeries for isthmic spondylolisthesis that combined ALIF + PLF surgeries demonstrated an 18.9% complication rate! Normally, the the complication rate for the posterior procedures is <10%.
What about decompression (laminectomy, laminotomy, foraminotomy)? Although simple decompression used to be the gold standard [75, 76], it just doesn't cut the mustard anymore, for this procedure does not address the frequent instability associated with isthmic spondylolisthesis which is thought to be a significant generator of low back pain.
*If you have jumped right to this page, it would be best to go back and read through the isthmic spondylolisthesis section first because much of that material and research directly relates to spondylolysis. Therefore, this section will be less comprehensive than the isthmic spondylolisthesis section.
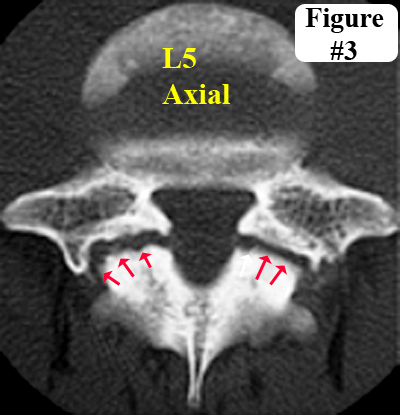 Spondylolysis is a potentially-painful condition of the spine that occurs when one or both of the pars interarticulari (plural) develop fractures (red arrows, figure 3), which are believed to be secondary to some sort of congenital weakness of that region.
Spondylolysis is a potentially-painful condition of the spine that occurs when one or both of the pars interarticulari (plural) develop fractures (red arrows, figure 3), which are believed to be secondary to some sort of congenital weakness of that region.
As previously mentioned, the majority of spondylolyses occur during the first decade of life [8] and frequently progress (~50% of them) into an isthmic spondylolisthesis by the age of 25. [8,9] Although they are usually asymptomatic, spondylolysis can produce chronic low back pain secondary to micro-motion within the fractured pars.
The prevalence of bilateral spondylolysis ranges from ~6% [8,20,45] to 11.5% in the normal population, [44] but greatly increases in children and young adults who participate in sports such as gymnastics, the throwing of events of track and field, and rowing. [51]
The most common symptom of spondylolysis is low back pain that is aggravated by motion at the waste—especially with sporting activity. Unlike isthmic spondylolisthesis, however, spondylolysis by itself does not typically cause concomitant (associated with) radicular pain or neurogenic claudication.
Like isthmic spondylolisthesis, however, spondylolysis is amenable non-operative care; however, for those patients who are refractory to such care, surgical stabilization of the pars fractures may be required.
Since there is no slip involved with the spondylolysis, there probably will not be any central or lateral recess stenosis. Therefore, no decompression (laminectomy, laminotomy, foraminotomy) would be needed, but one of the flavors of fusion would—possibly a PLF with instrumentation.
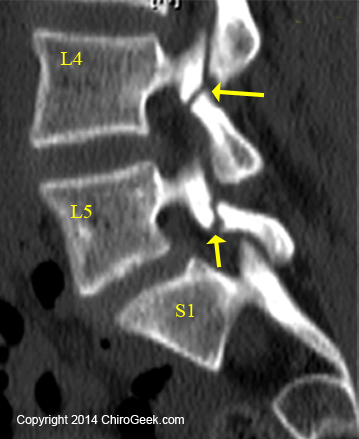 An isthmic spondylolisthesis almost always starts out as a bilateral (both sides) fracture [20,45] (i.e., a spondylolysis) of thinnest part of the posterior vertebral arch called the pars interarticularis (pars) (yellow arrows, left figure).
An isthmic spondylolisthesis almost always starts out as a bilateral (both sides) fracture [20,45] (i.e., a spondylolysis) of thinnest part of the posterior vertebral arch called the pars interarticularis (pars) (yellow arrows, left figure).
If such a fracture is present in one or both of the pars interarticulari (plural), yet no slip has occurred, then the condition is called a spondylolysis (figure left), which can be just as painful and debilitating as a spondylolisthesis.
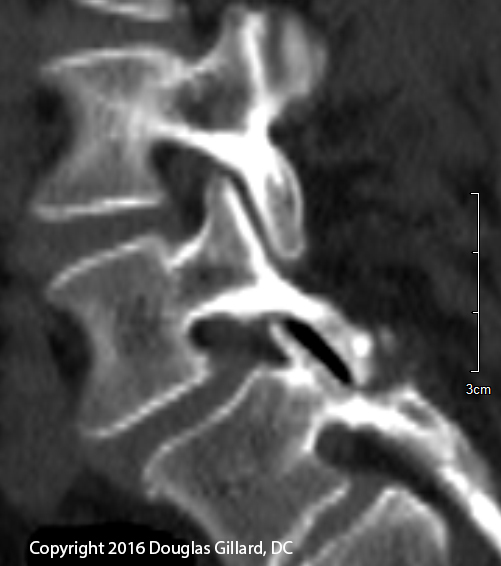
The figure left is a sagittal (from the side) computed tomographic (CT) image of the 22-year-old female who presented with severe chronic low back pain. The image reveals clear evidence of a non-displaced fracture (a fracture that has not separated very far apart) through both the pars interarticulari (plural) of L4 and L5 (yellow arrows); such findings lend support to the diagnosis of a bi-level spondylolysis. There might be a very slight anterior translation ("slip") of L5 on S1 (maybe 5%), in which case you could call this a spondylolisthesis.
These double level pars fractures are fairly rare and often do not respond to conservative care. This client ultimately ended up needing a two-level interbody fusion with decompression.
Unilateral (single-sided) spondylolysis (figure 0.75), which virtually never progresses into a slip and has a good chance to heal, [8,9] is a much rarer finding as noted by its prevalence of 0.8%. [8]
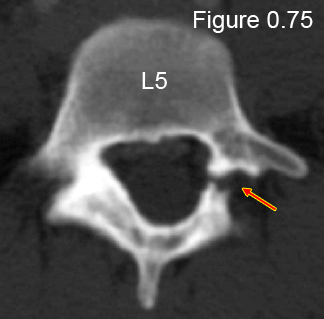 Figure 0.75: This is an axial CT image through the pedicles of L5 which clearly demonstrates a rare unilateral (one-sided) fracture through the pars (red arrow), which did not cause any discernible slip on the sacrum. Therefore, the diagnosis is unilateral spondylolysis of L5 on the sacrum.
Figure 0.75: This is an axial CT image through the pedicles of L5 which clearly demonstrates a rare unilateral (one-sided) fracture through the pars (red arrow), which did not cause any discernible slip on the sacrum. Therefore, the diagnosis is unilateral spondylolysis of L5 on the sacrum.
For patients with spondylolysis that was refractory to non-operative care, there are basically two surgical options: traditional lumbar fusion or direct repair.
Since the goal of fusion for spondylolysis is to stabilize the fractured posterior arch, typically a less invasive type of fusion is employed, such as posterolateral (PLF) fusion with instrumentation. Although instrumented PLF does not invade the epidural space (which is a very good thing), like the other types of fusion, it does destroy the normal biomechanics of the lumbar spine, which in turn overloads the adjacent vertebrae. Such an overload will make them vulnerable to new injury, chronic pain, and possibly the need for subsequent fusion—a phenomenon often referred to as "the domino effect."
In order to eliminate the domino effect, spine surgeons began trying to directly repair the fractured pars via several different methods, which fall into two broad categories: the outside wiring procedures and the internal screw fixation methods.
I will focus on internal screw fixation method, which really seems to be catching on.
A surgical technique called the Buck's procedure is one method for direct repair of a spondylolysis that has been gaining favor with some spine surgeons. [69]
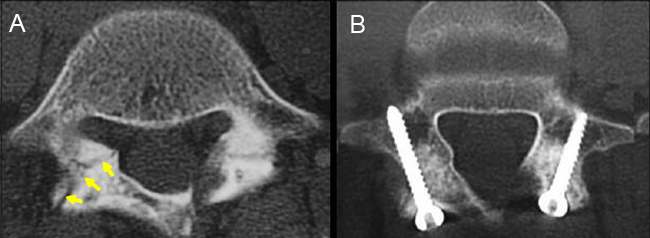 Although on paper the procedure looks quite logical and straightforward, in reality it is difficult to pull off, and the spine surgeon needs to be incredibly skilled in order to get the job done right.
Although on paper the procedure looks quite logical and straightforward, in reality it is difficult to pull off, and the spine surgeon needs to be incredibly skilled in order to get the job done right.
In a nutshell, the surgeon drills a long tiny hole through the narrow posterior arch and fractured pars. This is not an easy task because the posterior arch is quite thin and sometimes irregularly contoured. A CT-based monitoring technique called Stealth Technology should be used to ensure this hole is drilled just correctly. Then, after the fracture site is debride down to bleeding bone, iliac crest autograft (ICAG) (a small piece of your hipbone is taken and ground-up into a paste) will be placed in and around the fracture site (yellow arrows). The ICAG will act as cement and hopefully unite the broken ends of the pars together. Finally, a titanium screw will be threaded down the drilled hole, which will hold the broken pars together and allow for the ICAG to fuse everything into one unit. (Figure B)
Other direct repair techniques, often called the "cable screw technique" or "Scott's wiring technique," involves wiring the pars fracture site together instead of using the screws. However, I will not get into these techniques, for I believe they are on the way out. [68]
As expected with any new or rarely used technique, the spine literature does not contain any high-quality studies on this subject. Therefore, you must take the following with a grain of salt.
There was, however, one lower quality systematic review [66] that pooled the data from nine of the highest quality (only level III and IV evidence) direct repair papers (a mixture of Scott's wiring technique and Buck's procedure) and compared them with fusion (mainly PLF). After analyzing data from the two groups, Westacott et al. concluded that there was no statistical difference between direct repair and fusion with regard to clinical outcomes. [66] What do these results mean? Well, if true, they mean the patient can choose the Buck's procedure over fusion, which in turn will ostensibly preserve the lumbar spine biomechanics and eliminate the chance of suffering the post-fusion domino effect.
One of the pitfalls, however, with direct repair is that historically there has been a high rate of failure to fuse (non-union or pseudoarthrosis). In fact in one investigation, the rate of pseudoarthrosis was an unacceptable 25%. [67]
The Buck's procedure is one surgery where rH-BMP-2 might be considered, which would certainly reduce the chance of pseudoarthrosis. However, this osteobiologic has currently become controversial because of its weak association with cancer. [72] Therefore, this decision must be made with extreme caution and guidance from your primary treating physician.
Degenerative spondylolisthesis, which was first reported by German researchers back in the 1930s, [55] is a potentially painful condition of the spine that occurs when the degenerated facet joints of the affected vertebra fail to stop natural anterior translation, which in turn results a spondylolisthesis (a slip). It is differentiated from isthmic spondylolisthesis by the absence of fracture in the pars interarticularis or the posterior arch.
While isthmic spondylolisthesis is more a condition of youth, [8,9] degenerative spondylolisthesis is virtually never seen before the age of 40 years and considered more a condition of late middle age (>55 years). [57,59] Also unlike isthmic spondylolisthesis, people affected with degenerative spondylolisthesis virtually never have a slip that exceeds 30%. [57] In other words, there are no high-grade slips with degenerative spondylolistheses.
 Although not universally accepted, it is believed by some that a degenerative spondylolisthesis occurs because of an anomalous formation of the lumbar-sacral joint. Specifically, there is either not enough lordosis between L5 and the sacrum, or L5 is sacralized. [57] Such anatomy may result in a biomechanical overload of the L4 disc and facet joints, which in turn leads to significant degenerative changes of the facet joints and subsequent slip. [57]
Although not universally accepted, it is believed by some that a degenerative spondylolisthesis occurs because of an anomalous formation of the lumbar-sacral joint. Specifically, there is either not enough lordosis between L5 and the sacrum, or L5 is sacralized. [57] Such anatomy may result in a biomechanical overload of the L4 disc and facet joints, which in turn leads to significant degenerative changes of the facet joints and subsequent slip. [57]
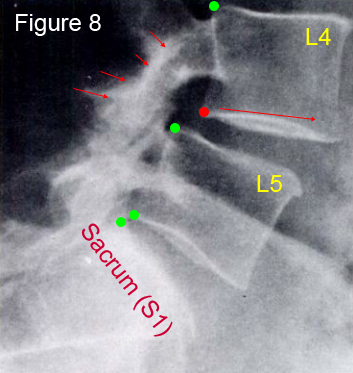
Figure 8 is a sagittal radiograph of a 62 year old female with a grade I degenerative spondylolisthesis at L4. Note the anterior translation (slip) of L4 on L5 and the intact pars interarticularis and posterior arch (short red arrows). (*if you're confused, please visit the forthcoming link to learn about naming spondylolisthses.) Therefore, you can tell this is a degenerative spondylolisthesis because there is no fracture line through the pars or posterior arch, as would be expected in an isthmic spondylolisthesis (figure 4). Furthermore, the slip is in an elderly female at L4 and does not exceed 30%; all of these findings point to the diagnosis of degenerative spondylolisthesis.
Jacobsen et al. [59] studied the prevalence (number of people with the condition) of degenerative spondylolisthesis in a population of over 4000 people (all of these folks had been x-rayed) and discovered that 6.3% of them, mostly females (5:1), had this condition. [59]
 They also discovered that there was no significant (p<0.05) relationship between degenerative spondylolisthesis and (1) the number of children a woman has had, (2) whether or not the woman was menopausal, or (3) whether or not the person suffered low back pain.
They also discovered that there was no significant (p<0.05) relationship between degenerative spondylolisthesis and (1) the number of children a woman has had, (2) whether or not the woman was menopausal, or (3) whether or not the person suffered low back pain.
On the other hand, increased age, lumbar lordosis, and BMI (how heavy you are) were all significantly related to the presence of degenerative spondylolisthesis. [59]
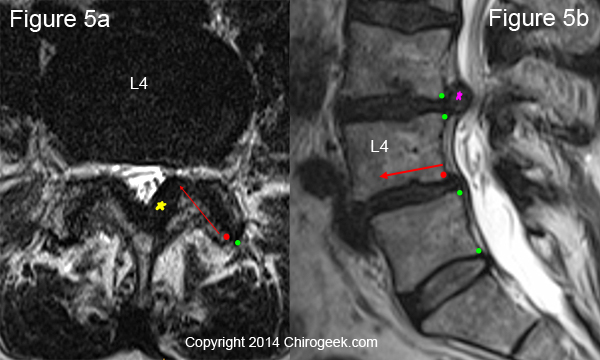
Figure 5 is an MRI image of a 66-year-old female with chronic low back pain and left-greater-than-right lower extremity pain. A careful inspection of the posterior arch (figure 5a) reveals no sign of fracture; however, there is a slip (long red arrow) in both facets as the inferior articular processes of L4 have slide forward (green vs red dot). The result of the slip and degenerative changes have caused left lateral stenosis within the lateral recess.
POP QUIZ: do you know what the pink and yellow stars are pointing out? The answers are a moderate disc herniation and severe ligamentum flavum hypertrophy, respectively.
Typically, since there is no fracture of the posterior arch, any low back pain experienced with this condition is thought to be secondary to irritated nerve fiber within the degenerated disc and/or facet joints.
If radicular pain or neurogenic claudication develop, then the cause is most likely secondary to stenotic-resulting degenerative changes of the ligamentum flavum, disc, facets, and/or the osseous boundaries of the lateral recess, and not solely the slip, which is almost always less than 30%. [57]
Just like spondylolysis and isthmic spondylolisthesis, people with degenerative spondylolisthesis often respond well to non-operative care (medication, physical therapy, low-force chiropractic care, acupuncture, lumbar bracing, facet injections, and/or epidural steroid injections). However, for patients who were refractory to such conservative treatment, surgical intervention may be needed. [70]
How do we know that surgery may work better then non-operative (aka, conservative care) for the treatment of degenerative spondylolisthesis that was refractory to conservative care? For the answer, we turn to the medical research:
In 2009, Weinstein et al. published the results of their investigation (which was part of the famous SPORT studies) that answered the question, "Does spine surgery outperform non-operative care?" [70] In order to answer the question, they followed over 300 patients, approximately half had conservative care and the other half underwent lumbar fusion, for four years and then reevaluated them. After reviewing all the data, they concluded that the patients who had undergone lumbar fusion demonstrated superior clinical outcomes with regard to the ability to function and pain levels, as compared to the patients who had only used non-operative care. [70]
Because there is no fracture to deal with in degenerative spondylolisthesis, many surgeons once believed that no lumbar fusion was indicated. Instead, a simpler decompression (aka, laminectomy, laminotomy, foraminotomy) was the way to go.
However, research has demonstrated that fusion (especially posterolateral fusion) in addition to decompression does statistically improve post-surgical patient outcomes when compared to decompression alone.
In a landmark and somewhat controversial study, Herkowitz et al. [71] prospectively studied the surgical outcomes of two groups of patients with lumbar degenerative spondylolisthesis with associated stenosis. Specifically, one group (n=25) was treated with laminectomy, and the other group (n=25) was treated with laminectomy plus non-instrumented posterolateral fusion (PLF). Both groups were carefully followed after surgery for a minimum of two years in order to see how they were doing. At the final follow-up, which averaged three years from the date of surgery, the patient's who had undergone fusion plus decompression demonstrated statistically superior clinical outcomes, as compared to the group that had only had decompression. The authors concluded by saying:
"The results of this prospective study clearly demonstrate that decompressive lumbar laminectomy with intertransverse process arthrodesis [PLF] is the operative procedure of choice for patients who have spinal stenosis associated with degenerative lumbar spondylolisthesis at a single level." [71]
Furthermore, I believe that it is not unreasonable to conclude that by the addition of instrumentation to the PLF, the results may have favorite fusion group even more because it has been previously demonstrated that the addition of such hardware reduces pseudoarthrosis, [48,49] and pseudoarthrosis can cause all sorts of problems.
On that note, let me present two fairly high-level-evidence systematic reviews that support the use of instrumentation with PLF and decompression for the treatment of degenerative spondylolisthesis.
In 1997, Fischgrund et al. [49] was awarded the prestigious Volvo Award for their paper that looked at the effectiveness of PLF as a treatment intervention for patients with degenerative spondylolisthesis. They also looked at whether or not the addition of posterior instrumentation (pedicle screws and rods) to the PLF procedure would enhance post-surgical clinical outcomes. The results indicated that good to excellent outcomes were achieved in 76% of the patients at the two-year follow-up. However, although instrumentation led to higher rates of successful fusion (which is a good thing), it did not improve clinical outcomes of these patients. [49]
In 2007, Martin et al. [48] published a systematic review (level II evidence) in the same prestigious Journal that also compared instrumented versus non-instrumented PLF to decompression alone as treatment interventions for degenerative spondylolisthesis. They concluded, as did Fischgrund [49], that decompression with PLF is better than decompression alone and that the posterior instrumentation increased the chances of successful fusion, but did not improve clinical outcomes. [48]
BOTTOM LINE: Based upon the research, it would appear that for patients who have failed conservative care, undergoing an instrumented fusion (probably PLF) and decompression as needed is the way to go. However, please be mindful that the very long-term results of fusion (>4 years) are severely understudied.
I hope you have enjoyed this page and, as always, please feel free to email me any questions or consider allowing me to go over your unique situation via my Coaching Service.
1) Leone LD, Lamont DW. “Diagnosis and treatment of severe dysplastic spondylolisthesis.” J AM Osteopath Assoc 1999;99:326-8
2) Wynne-Davies R, Scott JHS. “Inheritance and spondylolisthesis – a radiographic family survey.” J Bone Joint Surg Br 1997;618:301-5
3) Saha MM, et al. "Osteopetrosis with spondylolysis: four cases in one family." Br Radiol 43:738, 1970.
5) Wiltse LL. "Spondylolisthesis: Classification and Etiology." Symposium of the Spine, Am Acad Orthop Surg 1969; 143.
6) St. John TA, Alber TJ. "Reduction of Spondylolisthesis with Pedicle Screw Fixation And Transforaminal Lumbar Interbody Fusion." In Surgical Techniques for the Spine; Haher TR, Merola AA; Thieme – New York 2003.
7) Batts M Jr. "The etiology of Spondylolisthesis." J Bone Joint Surg (Am) 21:879, 1939
8) Fredrickson BE, Baker D, McHolick WJ, et al. The Natural History of spondylolysis and spondylolisthesis. J Bone Joint Surg (Am) 1984;66A:699-707.
9) Beutler WJ, Fredrickson BE, Murtland A, et al. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. 2003;28:1027-1035.
10) Stewart TD. "The Age Incidence of Neural Arch Defects in Alaskan Natives, Considered from the Stand Point of Etiology." J Bone Joint Surg (Am) 1953; 35:937
11) Kettelkamp DB, Wright DG. "Spondylolysis in the Alaskan Eskimo." J Bone Joint Surg (Am) 1971; 53:563.
12) Amundson G, et al. "Spondylolisthesis. In; Rothman RH, Simeone FA, eds. The Spine. 3rd ed. Philadelphia, PA, USA: WB Saunders; 1992:913-969.
13) Belfi LM, et al. "Computerized Tomography Evaluation of Spondylolysis and Spondylolisthesis in Asymptomatic Patients. Spine 2006; 31:E907-910.
16) Turner RH, Bianco AJ. "Spondylolysis and Spondylolisthesis in Children and Teenagers." J Bone Joint Surg (Am) 53:1298, 1971
17) Eisenstein SM, Ashton IK, Roberts S, Darby AJ, Kanse P, Menage J, Evans H. Innervation of the spondylolysis "ligament". Spine 1994; 19: 912-916.
19) Cyron BM, Hutton WC. The fatigue strength of the lumbar neural arch in spondylolysis. J Bone Joint Surg 1978; 60B: 234-238.
20) Belfi LM, Ortiz AO, Katz DS. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine 2006;31:E907-E910.
21) Hammerberg KW. New concepts on the pathogenesis and classification of spondylolisthesis. Spine 2005;30:S4-S11.
22) Cyron BM, Hutton WC, Troup JDG. Spondylolytic fractures. J Bone Joint Surg Br 1976;58:462-466.
23) Cyron BM, Hutton WC. The fatigue strength of the lumbar neural arch in spondylolysis. J Bone Joint Surg Br 1978; 60:234-238.
24) Green TP, Allvery JC, Adams MA. Spondylolysis: bending of the inferior articular process of the lumbar vertebrae during simulated spinal movements. Spine 1994; 19:2683-91.
25) Herkowitz HN. Spine update: degenerative lumbar spondylolisthesis. Spine 1995; 20:1084-1090.
26) Yochum TR, Rowe LJ. "Essentials of Skeletal Radiology" Baltimore; Williams & Wilkins; second edition: 1996:
27) Schmorl G, Junghans H. "The human Spine in Health and Disease, ed 2. New York, Grune & Stratton, 1971
31) Rowe GG, Roche MB. The etiology of separate neural arch. J Bone Joint Surg 1953; 35A: 102-110.
32) Hensinger RN. Spondylolysis and spondylolisthesis in children and adolescents. J Bone Joint Surg 1989; 71A: 1089-1107.
33) Bruske-Hohlfeld I, Merritt JL, Onofrio BM, et al. Incidence of lumbar disc surgery: a population-based study in Olmsted County, Minnesota, 1950-1979. Spine 1990;15:31-35.
34) O'Neill DB, Micheli LJ. Postoperative radiographic evidence for fatigue fracture as the etiology in spondylolysis. Spine 1989; 14: 1342-1355.
35) Wiltse LL, Widell EH, Jackson DW. Fatigue fracture: the basic lesion in isthmic spondylolisthesis. J Bone Joint Surg 1975; 57A: 17-22.
36) Wiltse LL, Newman PH, Macnab I. "Classification of spondylolysis and spondylolisthesis." Clin Orthop Relat Res. 1976 Jun;(117):23-9.
37) McKee BM, et al. "Spondylolysis and spondylolisthesis in Children: A Review." J Can Assoc Radiol 1971; 22:100
39) Wiltse LL, et al. "Classification of spondylolysis and spondylolisthesis." Clin Orthop 117:23, 1976
40) Moe JH, et al. "Scoliosis and other Spinal Deformities," Philadelphia, WB Saunders, 1978.
41) Macnab I. "Spondylolisthesis with intact an intact neural arch-so called pseudospondylolisthesis." J Bone Joint Surg (Br) 32:325,1950
42) Grobler L, Robertson P, Novotny J, et al. Etiology of spondylolisthesis. Assessment of the role played by lumbar facet joint morphology. Spine 1993;18:80-92.
43) Postacchini F, Cinott G. Bone regrowth after surgical decompression for lumbar spinal stenosis. J Bone Joint Surg [Br] 1992;74:862-869.
44) Kalichman L, Kim DH, Li L, et al. Spondylolysis and Spondylolisthesis. Spine 2009;34:199-205.
45) Sakai T, Sairyo K, Takao S, et al. Incidence of lumbar spondylolysis in the general population in Japan based on multidetector computed tomography scans from two thousand subjects. Spine 2009; 34:2346-2350.
46) Friberg S. "Studies on Spondylolisthesis." Acta Chir Scand 82(Suppl):55, 1939.
47) Wang SJ, Han YC, Liu XM, et al. Fusion techniques for adult isthmic spondylolisthesis: a systematic review. Arch Orthop Trauma Surg 2014; PMID:24715157 (ahead of print).
48) Martin CR, Gruszcynski AT, Braunsfurth HA, et al. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine 2007; 32:1791-1798.
49) Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo award in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine 1997; 22:2807-2812.
50) Toueg CW, Mac-Thiong JM, Grimard G, et al. Prevalence of spondylolisthesis in a population of gymnasts. Stud Health Technol Inform 2010;158:132-137.
51) Soler T, Calderon C. The prevalence of spondylolysis in the Spanish elite athlete. Am J Sports Med 2000; 28:57-62.
52) Saraste H: Long-term clinical and radiological follow-up of spondylolysis and spondylolisthesis. J Pediatr Orthop 1987; 7:631-638.
53) McCarroll JR, Miller JM, Ritter MA. Lumbar spondylolysis and spondylolisthesis in college football players: a prospective study. Am J Sports Med 1986; 14:404-406.
54) Kobayashi A, Kobayashi T, Kato K, et al. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med 2012; 41:169-176.
55) Junghanns H. Spondylolisthesis ohne spalt in zwischengelenstuck. Archiv fur Orthopaedische und Unfall-Chirurgie 1930;29:118-127.
56) Newman PH. Stenosis of the lumbar spine in spondylolisthesis. Clin Orthop 1976; 115:116-121.
57) Rosenberg NJ. Degenerative spondylolisthesis. J Bone Joint Surg [Am] 1975; 57:467-474.
58) Iguchi T, Wakami T, Kurihara A, et al. Lumbar multilevel degenerative spondylolisthesis: radiological evaluation and factors related to anterolisthesis and retrolisthesis. J Spinal Disord Tech 2002; 15:93-99.
59) Jacobsen S, Sonne-Holm S, Rovsing H, et al. Degenerative lumbar spondylolisthesis: in epidemiological perspective. The Copenhagen osteoarthritis study. Spine 2007;32:120-125.
60) Sengupta DK, Herkowitz HN. Degenerative spondylolisthesis: review of current trends and controversies. Spine 2005;30:S71-S81.
61) Yuan HA, Garfin SR, Dickman CA, Mardjetko SM. A historical cohort study of pedicle screw fixation in thoracic, lumbar, and sacral spinal fusions. Spine 1994;20S:2279S-2296S.
62) Gehrchen PM, Dahl B, Katonis P, et al. No difference in clinical outcomes after posterolateral lumbar fusion between patients with isthmic spondylolisthesis and those with degenerative disc disease using pedicle screw instrumentation. Eur Spine J 2002; 11:423-427.
63) Porter R. Spinal stenosis and neurogenic claudication. Spine 1996;21:2046-2052.
64) Rossi F, Dragoni S. Lumbar spondylolysis and sports. The radiological findings in statistical considerations. [Article in Italian] Radiol Med 1994;87:397-400.
65) Longo UG, Loppini M, Romeo G, et al. Evidence-based surgical management of spondylolisthesis: reduction or arthrodesis in situ. J Bone Joint Surg (Am) 2014;96:53-58.
66) Westacott DJ, Cooke SJ. Functional outcome following direct repair or inter vertebral fusion for adolescent spondylolysis: a systematic review. J Pediatr Orthop B 2012;21:596-601.
67) Pai VS et al. Repair of spondylolytic defect with a cable screw reconstruction. Int Orthop 2008;32:121-125.
68) Kip PC, et al. Biomechanical testing of pars defect repairs. Spine 1994; 19:2692-2697.
69) Rajasekaran S, et al. Direct repair of lumbar spondylolysis by Buck's technique. ndian J orthop 2011;45:136-140.
70) Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with non-operative treatment for lumbar degenerative spondylolisthesis: four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg (Am) 2009;91:1295-1304.
71) Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg 1991;73:802-808.
72) Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011;11:471-491.
73) Wiltse LL, Newman PH, MacNab I. Classification of spondylolysis and spondylolisthesis. Clin. Orthop 1976; 117:23-29.
74) Thirukumaran CP, et al. National Trends in Surgical Management of Adult Lumbar Isthmic Spondylolisthesis: 1998 to 2011. Spine 2016; 41:490-501.
75) Gill GG, et al. Surgical Treatment of Spondylolisthesis without Spine Fusion; Excision of the Loose Lamina with Decompression of the Nerve Roots. J Bone Joint Surg Am 1955; 37-A: 493-520.
76) Gill GG. Long-Term Follow-Up Evaluation of A Few Patients with Spondylolisthesis Treated by Excision of the Loose Lamina with Decompression of the Nerve Roots without Spinal Fusion 1984; 182:215-219.
77) Moller H, et al. Instrumented and Non-Instrumented Posterolateral Fusion in Adult Spondylolisthesis: a Prospective Randomized Controlled Trial: Part#2. Spine 2000; 25:1716-1721.
78) Swan J, et al. Surgical Treatment for Unstable Low-Grade Isthmic Spondylolisthesis in Adults: a Prospective Controlled Study of Posterior Instrumented Fusion Compared with Combined Anterior-Posterior Fusion. Spine J 2006; 6:606-614.
79) Kwon BK, et al. A Critical Analysis of Literature regarding Surgical Approach and Outcome for Adult Low-Grade Isthmic Spondylolisthesis. J Spinal Disord Tech 2005; 18(supp):S30-S40.
80) Liu XY, et al. Meta-Analysis of Circumferential fusion versus Posteriolateral Fusion in Lumbar Spondylolisthesis. J Spinal Disord Tech 2014; 27:E282-293.
126) Bogduk N, McGuik B. "Medical Management of Acute & Chronic Low Back Pain." vol 13 Amsterdam; Elservier: 2002
131) Roche MB. "The pathology of Neural-Arch Defects." J Bone Joint Surg 1949: 31A: 529-537
132) Ghelman B, Doherty JH. "Demonstration of Spondylolysis by Arthrography of the Apophyseal Joint." Am J Roentgenol 1978; 130:986-987.
133) Schneiderman GA, et al. "The Pars Defect as a Pain Source: a Histological Study." Spine 1995; 20:1761-1764. "CONCLUSIONS. Free nerve endings believed to have nociceptive function were identified in all specimens. The finding of neural elements, including free nerve endings within the pars defect tissue, suggests that the pars defect may be a source of back pain in some patients with symptomatic spondylolysis."
134) Eisenstein SM, et al. "Innervation of the Spondylolysis 'Ligament'. Spine 1994; 19:912-916. "CONCLUSION. Spondylolysis of the lower lumbar vertebrae is a non-united childhood fracture of the arch of the vertebra, persisting into adult life. Symptoms of disabling low back pain appear in a minority of patients, usually for the first time in adulthood. This pain is considered to arise from several separate sources, one of which may be the spondylolysis ligament. The movement that the ligament allows at the fracture site may result in stimulation of the nerve endings both in the ligament and in the surrounding soft tissue."
135) Cyron BM, Hutton WC. "Variations in the amount and Distribution of Cortical Bone Across the Partes Interarticulares of L5. A predisposing Factor in Spondylolysis?" Spine 1979; 4: 163-167
136) Cyron BM, Hutton WC. "The Fatigue Strength of the Lumbar Neural Arch in Spondylolysis." J Bone Joint Surgery 1978: 60B:234-238.
138) Green TP, et al. "Spondylolysis: Bending of the Interior Articular Processes of Lumbar Vertebrae during Simulated Spinal Movements." Spine 1994; 19: 2683-2691.
139) Farfan HF, et al. "The Effects of Torsion on the Lumbar Intervertebral Joints: The role of Torsion in the production of Disc Degeneration." J Bone Joint Surg 1970; 52A: 468-497
150) Hensinger RN. "Spondylolysis and Spondylolisthesis in Children and adolescents." J Bone Joint Surg 1989; 71A:1089-1107
152) O'Neil DB, Micheli LJ. "Postoperative Radiographic Evidence for Fatigue Fracture as the Etiology in Spondylolysis." Spine 1989; 14:1342-1355
153) Wiltse LL, et al. "Fatigue Fracture: the Basic Lesion in Isthmic Spondylolisthesis." J Bone Joint Surg 1975; 57A: 17-22
154) Suh PB, Esses SI, Kostuik JP. "Repair of pars interarticularis defect. The prognostic value of pars infiltration." Spine. 1991; 16(8 Suppl):S445-8. "Ten patients with symptomatic spondylolysis or Grade I spondylolisthesis were treated with the Buck method. At follow-up, nine patients were graded as successful. All patients fused. Pain relief, level of function, and likelihood of return to work were higher in patients preoperatively selected by lidocaine infiltration of the pars defect. Pars infiltration gives an accurate prediction of successful outcome following pars repair."
204) Batts, Martin, Jr. "The Etiology of Spondylolisthesis." J Bone and Joint Sur. 1939; 21:879-884
208) Friberg, Sten: "Studies on Spondylolisthesis." Acta Chi. Scandinavica, Supplementum 55, 1939,
211) Hitchcock HH. "Spondylolisthesis. Observations on Its Development, Progression,
and Genesis." J Bone and Joint Surg.; 1940; 22:1-16
224) Rowe GG, Roche MB. "The Etiology of Separate Neural Arch." J Bone and Joint Surg; 1953; 35-A:102-110
240) Wiltse LL. "Etiology of Spondylolisthesis." Clin Orthop 1957; 10:48-59.
241) Wiltse LL. "Spondylolisthesis in Children." Clin Orthop 1961; 21:156-163.
242) Wiltse LL. "The Etiology of Spondylolisthesis." J. Bone and Joint Surg 1962 44-A:539-560
244) Wiltse LL. "Fatigue Fracture: The Basic Lesion in Isthmic Spondylolisthesis." J Bone and Joint Surg 1975; 57-A: 17-22
245) Wynne-Davies, et al. "Inheritance and Spondylolisthesis. A radiographic Family Survey." J Bone and Joint Surg 1979; 61-B(3); 301-305
500) Professor Nikolai Bogduk, MD, Multiple Volvo Award Winner ‘Evidence-Based Clinical Guidelines for the Management of Acute Low Back Pain’ The Australasian Faculty of Musculoskeletal Medicine November 1999; Chapter 9
501) Libson E, Bloom RA, Dinari G. Symptomatic and asymptomatic spondylolysis and spondylolisthesis in young adults. Int Orthop 1982;6:259-261.
502) Beck RW, Holt KR, et al. "Radiographic anomalies that may alter chiropractic intervention strategies found in a New Zealand population." J Manipulative Physiol Ther. 2004 Nov-Dec;27(9):554-9. Conclusions: " Eight hundred forty-seven full-spine radiographs were included in the study. Anomalies were found in 68% of patients who had radiographs taken. The 5 most frequently occurring anomalies in descending order were degenerative joint disease (23.8%), posterior ponticle (13.6%), soft tissue abnormalities (13.5%), transitional segments (9.8%), and spondylolisthesis (7.8%). Other noteworthy occurrences because of their generalized status as absolute contraindications to adjustment are fracture (6.6%), malignant tumor (0.8%-3.1%), abdominal aortic aneurysm (0.8%) and atlantoaxial instability (0.6%)."
503) Moreton RD. "Spondylolysis." JAMA. 1966 Feb 21;195(8):671-4. " In 32,600 asymptomatic adults, the prevalence of a pars defect was found to be 7.2%."
512) Jensen MC, et al. “MRI imaging of the lumbar spine in people without back pain.” N Engl J Med – 1994; 331:369-373
[ Top ]
© Copyright Protected, 2002 - 2013 All rights reserved - Douglas Gillard DC.