The Stenosis Page |
 |
Note: It would behoove the reader to visit the Disc Anatomy Page in order to freshen up on the basic anatomy of the motion segment before continuing on, for I will make the assumption he or she know a little bit about it. Because I often use real MRI images as teaching tools, the reader might also visit The MRI Page to freshen up on that topic as well.
Last updated: 8/14/16
We have already learned that compression and inflammation of a lumbar nerve root can cause chronic lower extremity pain and, indirectly, low back pain secondary to conditions such as disc herniation and spondylolisthesis.
Now, let's learn about another condition that can also compress and inflame the lumbar nerve roots.
 What is Lumbar Spinal Stenosis?
What is Lumbar Spinal Stenosis?In simple terms, lumbar spinal stenosis (LSS) is a condition that occurs when the passageways within the vertebral column become narrowed and constrict the nerve roots contained within them. Such a constriction may, but not always, [25,95] result in disabling lower extremity pain, limited ability to walk and stand, and, indirectly, severe lower back pain.
Perhaps surprisingly, LSS is the most common cause for spine surgery in patients over the age of 65, and its prevalence (how often it is seen in a population) is on the rise. [24] In fact, it has been predicted that over 64 million elderly adults will be affected by this disabling condition over the next decade. [14,15,34]
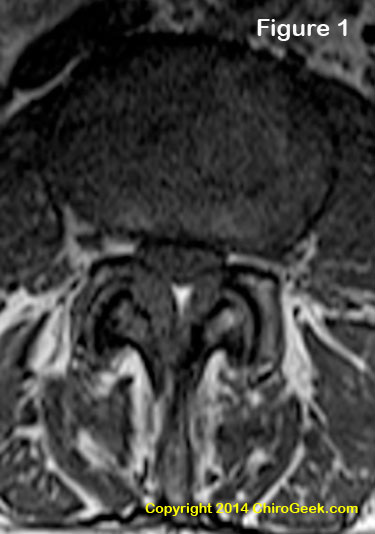
Figure 1 is an axial T1-weighted MRI image through the L3 disc space that demonstrates moderate to severe central stenosis secondary to severe ligamentum flavum thickening, facet joint hypertrophy, and disc bulging. In this case, the nerve root compression resulted in patient symptoms of neurogenic claudication (a limited ability to walk) secondary to a squeezing of the thecal sac, which in turn resulted in ischemia and axon injury. Can't see what I'm talking about? Click here for some help.
Of all the diagnoses for chronic back and leg pain, LSS is perhaps the most feared by clinicians and patients alike. This is because not only is LSS difficult to diagnose, [13,19,20] it typically does not respond well to conservative care, which in turn may force the patient in to surgery. To that end, one recent medical study reported that 21% of patients with the new diagnosis of LSS necessitated (needed) spine surgery within three years from the date of that diagnosis. [33] In other words, almost one quarter of patients newly diagnosed with stenosis end up on the operating table within three years!
Although developmental anomalies (problems from birth) of the spine may account for some cases, LSS is typically an acquired condition that results from degenerative changes within the spine, such as disc bulging, ligamentum flavum thickening, and/or facet joint hypertrophy (bony thickening). Specifically, any one of these factors, alone or in combination, may narrow the passageways within the lumbar spine and crush the nerve root(s) contained within. (figure 1) Once a nerve root is compressed by a certain magnitude of force and for a certain duration of time, it can turn that nerve into a chronic pain generator, which the patient experiences as radicular pain and/or lower extremity dysfunction.
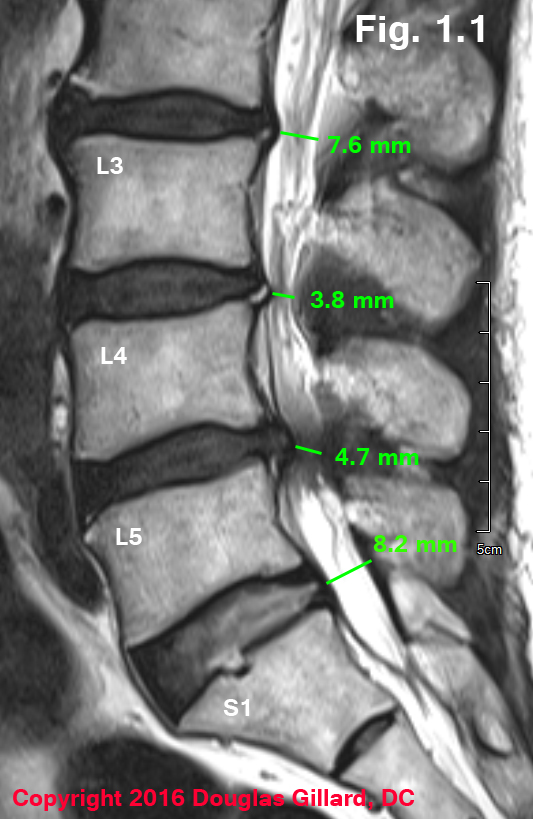 Figure Left: 75-year-old female with severe intermittent neurogenic claudication (can't walk for more than five minutes without lower extremity pain). Mid-sagittal MRI demonstrates severe central stenosis secondary to congenitally short pedicles (not visualize), ligamentum flavum thickening and disc bulging/herniation. Depending on the source, a vertebral canal that is < 13 mm and midsagittal MR or CT is considered central stenosis.
Figure Left: 75-year-old female with severe intermittent neurogenic claudication (can't walk for more than five minutes without lower extremity pain). Mid-sagittal MRI demonstrates severe central stenosis secondary to congenitally short pedicles (not visualize), ligamentum flavum thickening and disc bulging/herniation. Depending on the source, a vertebral canal that is < 13 mm and midsagittal MR or CT is considered central stenosis.
Because physical examination findings have been found to be almost useless for the purpose of diagnosing a patient with LSS, [19] the healthcare provider must rely upon special studies (imaging, electrodiagnostics, and diagnostic injections) and patient complaints to get the job done. [5,19] Hands down, magnetic resonance imaging (MRI) is the new gold standard (the best test available) with regard to making the diagnosis of LSS. Specifically, in 2013, de Schepper et al. [16] reported the results of their systematic review (one of the highest levels of evidence we have) which concluded that MRI was superior for diagnosing LSS when compared to electromyography (EMG), nerve conduction velocity (NCV), computed tomography, (CT) and even myelography, which was the old gold standard. [16]
The other component for making the diagnosis of LSS are patient symptoms. Specifically, there are two key symptoms that are the hallmark for the diagnosis: the first is a bilateral (both sides) lower extremity pain, heaviness, and cramping, which is brought on by walking or standing for a certain amount of time. The second, which is related to the first, is the almost miraculously disappearance of those lower extremity symptoms after sitting or bending forward at the waist for short time. [16,19] The aforementioned stenotic walking condition was given the name neurogenic claudication (a.k.a.: intermittent claudication) and must be differentiated from vascular claudication, peripheral vascular disease, and peripheral neuropathy, [5] which is beyond the scope of this page. We shall discuss neurogenic claudication in greater depth farther below.
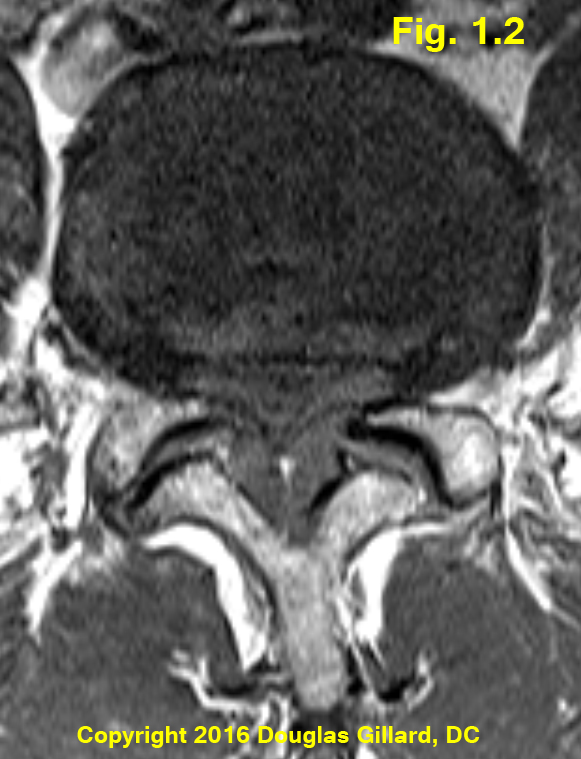 Figure 1.2 demonstrates severe central stenosis secondary to severe ligamentum flavum hypertrophy, a general bulging of the degenerated disc, and a small central disc protrusion. This patient was unable to walk more then five minutes without the development of severe lower extremity pain (this phenomenon is called intermittent neurogenic claudication) which forced him to sit down in order to gain relief. The patient will soon undergo decompressive surgery in hopes of increasing his ability to walk.
Figure 1.2 demonstrates severe central stenosis secondary to severe ligamentum flavum hypertrophy, a general bulging of the degenerated disc, and a small central disc protrusion. This patient was unable to walk more then five minutes without the development of severe lower extremity pain (this phenomenon is called intermittent neurogenic claudication) which forced him to sit down in order to gain relief. The patient will soon undergo decompressive surgery in hopes of increasing his ability to walk.
As we shall learn farther below, the patient may experience a unilateral (on one side) radicular pain secondary to a condition called lateral stenosis, which has a high rate incidence at L5/S1.
Lateral stenosis-related radicular pain is often difficult to differentiate from disc-herniation-related radicular pain.
Lateral stenosis occurs when then either the lateral recess canal and/or the neural foramen become narrowed from osteoarthritic change or spondylolisthesis/spondylolysis and compress the involve nerve root with such magnitude and duration that axons within the nerve root die, which in turn sends pain signals to the brain.
As with any pain-generating condition of the spine, nonoperative (conservative) care is typically the first choice of treatment for LSS and includes rest, bracing, exercise, activity modification, physical therapy, chiropractic care, and acupuncture. Injective procedures such as epidural steroid injections may also be considered as either an adjunct to conservative care, or a secondary treatment used after the failure of conservative care.
 An interspinous process distraction devices (ISPDD, interspinous device, ISD, interspinous spacer, or ISS) or a is a piece of hardware that is implanted surgically between adjacent spinous processes in such a way that it forces that motion segment (vertebra, disc, vertebra) into flexion. In other words, get "jacks-up" the back of the two adjacent vertebrae which results in a widing of the bony holes that are compressed in symptomatic spinal stenosis.
An interspinous process distraction devices (ISPDD, interspinous device, ISD, interspinous spacer, or ISS) or a is a piece of hardware that is implanted surgically between adjacent spinous processes in such a way that it forces that motion segment (vertebra, disc, vertebra) into flexion. In other words, get "jacks-up" the back of the two adjacent vertebrae which results in a widing of the bony holes that are compressed in symptomatic spinal stenosis.
Not only does the device open-up the compressed holes, it also prevents backwards bending (extension) of the motion segment which helps prevent flareups of pain and further injury to the nerve root.
There are several types of these interspinous devices, which include X-Stop, X-Stop PEEK, Coflex, IPD, DIAM, and APERIUS; arguably, the former two are the most popular.
The X-Stop was approved by the FDA in November 2005 and the X-Stop PEEK, in 2006. It is a proof for the treatment of patients over the age of 49 who suffer from neurogenic intermittent claudication secondary to confirm lumbar spinal stenosis and to have failed at least six months of nonoperative care. It is also only approved for two lumbar levels (motion segments).
Coflex was approved in 2012 and has added contraindications including prior fusion or laminectomy at the index level (planned-level of insertion), lumbar compression fracture, morbid obesity, and severe facet joint hypertrophy. there are also a lot of other FDA warnings with this device that you should read on your own here:
Key contraindications for the use of interspinous devices include significant motion segment instability (i.e., spondylolisthesis greater than grade I); acute fractures of the spinous process or pars interarticularis; moderate scoliosis (Cobb > 25°); cauda equina syndrome; severe osteoporosis in the spine or hip there is more than 2.5 standard deviations; or local infection.
Anecdotally, I have never recommended these devices because of a very high rate of revision surgery. This anecdotal recommendation is now supported by quality research.
THE RESEARCH:
Wu et al. interspinous spacer versus traditional decompressive surgery for lumbar spinal stenosis: a systematic review and meta-analysis. PloS ONE 2014; 9:e97142.
In 2014, Wu et al published the results of their systematic review and meta-analysis (the highest level of scientific evidence) which compared the clinical outcomes, complication rates, and revision surgery rates of interspinous devices versus traditional decompressive surgery, the latter of which is still considered the gold standard treatment for neurogenic intermittent claudication secondary to central stenosis.
After looking through 426 potential studies, only five were of very high quality; the patients and data of these five studies were pooled together and analyzed.
Bottom Line: although the surgical complications and clinical outcomes at 1-2 years status post surgery were not statistically different between the groups, the rate of reoperations (i.e., revision surgery) was over 3 times higher in the interspinous device group when compared to the traditional decompression surgery group. specifically, 23% (!) of the interspinous spacer group needed revision surgery, versus a more reasonable 6.9% in the traditional decompression group. [40]
Other researchers discovered similar findings: Bowers et al found that interspinous device surgery carried a high rate of complication which was later confirmed by Kim et al. [10,11]
Comments: the most surprising discovery following my research digging on this subject was that there were no high quality outcomes studies that followed these surgically implanted interspinous devices for more than a few years! Why? I would speculate because the rate of revision surgery is an enormous! (It's already 23% at two years)To support my speculation, I offer this: interspinous devices have been approved by the FDA for 10 years now and yet we have not a single high quality study that has followed the outcomes of these devices for more than two years! I would bet anything that the stakeholders have been following the outcomes of these original studies and realized that they are ending up with huge revision surgery rates and therefore decided not to do any follow-up publication on the original studies. What other exclamation could it be? this sort of thing is called "publication bias."
As a last resort, spinal surgery may be considered if nonoperative and injective care have failed to adequately control the symptoms of LSS. The procedure of choice is usually a decompression (i.e., laminectomy, laminotomy, facetectomy, medial facetotomy, foraminotomy, with or without discectomy). This procedure attempts to surgically "open up" or decompress the constricted vertebral canal, lateral recesses, and/or neuroforamina.
*Although the rate of lumbar fusion for the treatment of LSS has significantly risen over the last decade, [8] a recent large study (n=5,390) out of Sweden failed to demonstrate the superiority of fusion when compared to the much less invasive and cost-efficient decompression. [21] Therefore, one should perhaps be wary of a surgeon who offers fusion for an uncomplicated case of LSS, instead of a decompression.
Furthermore, in 2016, Forsth et al published a randomized controlled trial (one of the highest levels of evidence) in the prestigious New England Journal of Medicine which demonstrated that clinical outcomes at two years and even five years status-post surgery were not statistically different between decompression alone versus decompression + fusion, for the treatment of one or two level severe spinal stenosis! [Forsth et al. N Engl J Med 2016; 374:1413-1423.]
With regard to effectiveness, decompression may in fact work fairly well for decreasing the pain associated with LSS and has even been found superior to conservative care in several high-quality investigations. [1-4] However, like conservative care, fusion is less successful at increasing the post-operative walking distance for those patients affected severely with pre-operative neurogenic claudication. [22]
The Timing of Surgery: The timing of surgery (i.e., how long should a patient suffer before surgery is tried) for LSS has also been studied, and, just like the timing of microdiscectomy for disc herniation, was found to be very important. Specifically, researchers demonstrated that for patients who have failed conservative care, it is best to move ahead with surgery quickly (i.e., no later then 12 months after the onset of severe lower limb symptoms). Otherwise, at least according to this study, the chances of having a successful stenotic decompression surgery will be diminished. [23] In other words, the sooner you remove the nerve root compression via a surgical decompression, the better the chances for clinical outcomes (how good the patient believes he or she did following the surgery). [23]
*Warning: Making the diagnosis of LSS and deciding on a treatment plan is extremely complicated, for every individual presents with unique and sometimes complicating factors. Do not attempt to diagnose yourself or make a decision regarding surgery without first discussing it with your primary health care provider or specialist!
I would be more than happy to speak with you on the phone in order to help solve your unique puzzle of pain.
Please read about my Coaching Service and look at the Patient Testimonials regarding that service. There is no doubt that I will teach you a thing or two about your condition and help you formulate a treatment algorithm based upon my experience as a professional spine researcher, author, and clinician with over 20 years of experience.
Okay, so much for the general introduction. Now let's really dig into this topic.
The rest of this section is really geared for the spine surgeon, PM&R physician, or other healthcare provider with an interest in spine, including medical and chiropractic students. However, the layperson should be able to grasp the general concepts here.
There are generally two types of spinal stenosis: central stenosis or lateral stenosis, which will be discussed in depth farther below.
Although the first surgery for LSS was reported in 1893, [11] the diagnosis wasn't well accepted by the surgical community until the 1954 publication of a seminal paper by the Dutch neurosurgeon, Henk Verbiest. [13] In that paper, he explained how a degenerative narrowing of the central spinal canal could result in a pain-generating nerve root compression and how the diagnose of LSS could be made with CT myelogram and cetain key patient symptoms. Verbiest also described the nuances of stenosis surgery in seven of his patients. [13]
Although we have greatly improved our ability to diagnose and treat LSS, the overall diagnostic and treatment algorithms are the same: find the area(s) of nerve root compression via diagnostic testing and key patient symptoms; surgically decompress these areas, if nonoperative care failed; and then hope for the best.
*For you medical students who want to become spine surgeons or PM&R doctors, it is imperative that you understand the anatomical nuances of the lumbar spine. Let's get started.
Surprisingly, the medical literature continues to be ununited and, quite frankly, a "mess" with regard to terminology and the anatomical description of the important nerve root passageways within the lumbar spine. [5,26,28,31,36,38,39] However, after my thorough study the aforementioned books and papers, most of which describe the pathoanatomy of LSS, a single logical model has arisen which I shall use in this section.
The lumbar motion segments (a vertebra-disc-vertebra complex) of the lumbar spine have three canals/regions where nerve roots are vulnerable to bony/ligamentous compression: #1) the central canal (aka: spinal canal, central spinal canal, or vertebral canal), #2) the nerve root canal (aka: lateral recess canal, or subarticular canal, to name a few), and #3) the neural foramen (a.k.a. intervertebral foramen or IVF). *There is also an extra foraminal zone [26] where far out compression syndromes may occur on rare occasions. I will not be discussing them on this page.
Let's talk about each of these canals.
Let's start the discussion of the central canal by looking at a T1-weighted disc-level MRI cut through the L3/L4 motion segment. (figure 3)
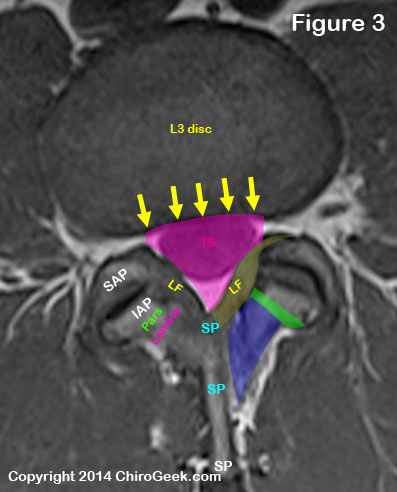 The central canal (pink outline) is basically a large hole in the center of the lumbar lumbar spine that houses the free-hanging lumbar nerve roots (collectively called the cauda equina) within a sac of the dura mater, which is called the thecal sac (aka: dural sac) (TS).
The central canal (pink outline) is basically a large hole in the center of the lumbar lumbar spine that houses the free-hanging lumbar nerve roots (collectively called the cauda equina) within a sac of the dura mater, which is called the thecal sac (aka: dural sac) (TS).
The central canal is bordered anteriorly by the posterior vertebral body (not seen on this cut) and intervertebral disc (yellow arrows).
Typically there is not much of a true posterior border, but if you had to pick one, then it would be the ligamentum flavum which is situated on top of the spinous process base (SP).
The posterolateral borders of the central canal are the ligamenta flava (plural, yellow, LF) which covers the lamina (blue) , pars interarticularis (pars, green), inferior articular process (IAP) and superior articular processes (SAP).
The facet joint capsule (not shown) is also part of the posterior lateral border.
*Click here for a clean view of figure 3, without markup.
The lateral border of the central canal is formed by the medial portion of the pedicle (or beginning of the intervertebral foramen) and a little bit of the superior articular processes in the mid and upper lumbar vertebra (L1/L2 - L4/L5), which is not seen on disc-level imaging.
Now let's take a look at the central canal and thecal sac (TS) two cuts below the disc-level view we saw. (figure 4)
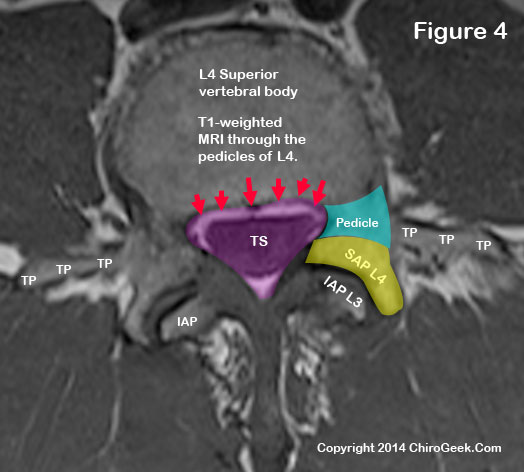 *If I just lost you by talking about "cuts," then you need to go back to the MRI page for further study :-).
*If I just lost you by talking about "cuts," then you need to go back to the MRI page for further study :-).
In figure 4, we can now see the lateral central canal borders in better detail. Note the pedicle (blue) and superior articular processes (yellow) clearly act as a border for the central canal (the central canal is outlined in pink).
We can also see, for the first time, the posterior vertebral body margin border (red arrows), which borders the anterior central canal.
The posterolateral borders are the same in this cut: lamina, pars interarticularis, inferior articular processes, and the superior articular processes.
As a side note, in this view you can actually see the transverse processes (TP), which are incredibly important in surgical procedures such as posterolateral fusion.
Note that the inferior articular processes are not fully formed in this view, which allows the lamina to act as a full border at the posterolateral region of the central canal. *Click here for a clean view of figure 3, without markup.
The nerve root canal was, perhaps surprisingly, best described by Bose [38] and Rauschning [39] back in the 1980s from their dissection of 93 fresh cadaver spines. The canal runs obliquely downward, medially to laterally, from the beginning of the dural sleeve (the point on the thecal sac where the nerve root laterally buds off) to the beginning of the neural foramina.
Although many have attempted to subdivide the nerve root canal into different zones (an entrance, mid, interpedicular, exit, and extra foraminal zone) [26,31], such subclassifications are really not a good way to describe it and in fact are downright confusing. This is because the so-called entrance zone really does not exist consistently above the L5/S1 motion segment. [36,38,39]
So with the exception of L5/S1, the nerve root canal is really nothing more than a bony trough or groove that is found on the ~anterolateral inferior surface of the superior articular processes, pars interarticularis, lamina [36] and/or pedicle, which funnels the traversing nerve root from its takeoff point on the thecal sac, to the neural foramen at the same level.
Thing are almost always different, however, at the L5/S1 motion segment, for the S1 nerve root buds-off the thecal sac above the L5 disc and then "traverses" obliquely downward adjacent to the L5 posterior disc where it is vulnerable to disc herniation. [39] Let's talk about it specifically.
I am comfortable describing the L5/S1 nerve root canal as having two zones: an entrance zone and a mid-zone.
The entrance zone begins at the takeoff point of the S1 nerve root from the thecal sac, which typically occurs at the L5 inferior vertebral body level (approximately at the inferior endplate of L5 and above is the disc). The nerve root then courses obliquely downward and in close proximity to the posterior L5 disc. Most of the way down, it has no true medial or lateral border until it reaches the superior articular facet and then inferior articular facet. It dives into the mid-zone at the base of the superior articular facet and then jumps in a bony groove within the lamina or pars interarticularis (everyone is a little different) where it is funneled around the medioinferior pedicle and into the neural foramina.
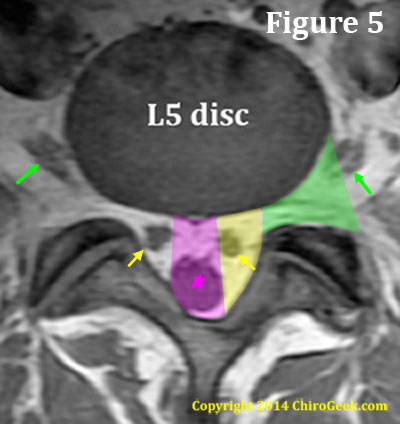 Figure 5. Because of this extra entrance zone, the axial anatomy at the L5 disc level is different than the other lumbar disc levels. Specifically, instead of two visible zones (the central canal and the neural foramina) there are three: the central canal, lateral recess (which is really the entrance zone of the lumbar nerve root canal) and the neural foramina ( you could call this the neural foramina zone if you wish).
Figure 5. Because of this extra entrance zone, the axial anatomy at the L5 disc level is different than the other lumbar disc levels. Specifically, instead of two visible zones (the central canal and the neural foramina) there are three: the central canal, lateral recess (which is really the entrance zone of the lumbar nerve root canal) and the neural foramina ( you could call this the neural foramina zone if you wish).
The central canal (pink) is bordered anteriorly by the disc and posteriorly by ligamentum flavum, which is covering the base of the spinous process and proximal laminae. It's lateral borders are defined by the size of the thecal sac and will vary. In figure 5, the patient had a small thecal sac; therefore, the central canal is quite narrow.
The lateral recess (yellow) is bordered medially by the central canal and posterolaterally by the lamina, pars interarticularis, inferior articular processes, and superior articular processes, as well as the pedicles of the neural foramina. It's anterior border is also the posterior disc.
The neural foramen (green) is the bony hole through which the exiting nerve root leaves the spinal column. It is bordered anteriolaterally by the disc and vertebral bodies (not shown on this cut), medially by the medial edge of the superior and inferior pedicles, posteriorly by the superior articular processes, and laterally by an imaginary line drawn from the outermost portion of the superior articular process/facet to the lateral disc.
As noted above in figure 5, The neural foramen (green) is the bony hole through which the exiting nerve root leaves the spinal column. It is bordered anteriolaterally by the disc and vertebral bodies (not shown on this cut), medially by the medial edge of the superior and inferior pedicles, posteriorly by the superior articular processes, and laterally by an imaginary line drawn from the outermost portion of the superior articular process/facet to the lateral disc.
The neuroforamen contains the dorsal root ganglion (DRG) of the sensory root 59% of the time. [31] 8% of the time, the DRG is found lateral to the neural foramen (in the extraforaminal zone) and 33% of the time it is medially located. [31]
Okay, I hear you, enough of this anatomy stuff. Let's get into what causes stenosis.
Central stenosis, which is the most common form of LSS, [25,31,95] is a condition that occurs at the disc level when the central spinal canal becomes significantly narrowed secondary to osseous (60% of the time) and/or ligamentous (40% of the time) thickening, [31] which is typically secondary to degenerative change. More specifically, the diagnosis is made when the axial mid-diameter of the canal becomes narrowed to a measure less than 13 mm, a condition called relative stenosis, [7-9] or less than 11 mm, a condition called absolute stenosis. [7-9] The prevalence of absolute versus relative stenosis has been reported 4.8% and 23.6%, respectively. [7]
Degenerative change is not always the cause of stenosis. Technically speaking, stenosis has been classically divided into two subcategories: congenital-developmental stenosis and acquired stenosis. [30,31]
Congenital-developmental stenosis occurs secondary to anomalous (idiopathic) vertebra formation or from dwarfism (achondroplasia).
Acquired stenosis, which accounts for the lion's share of the condition, is broken down into the following categories: degenerative (the most common); combined congenital and acquired; spondylolytic or spondylolisthesis; iatrogenic (e.g., post decompression surgery); posttraumatic; and metabolic, from conditions such as Paget's disease, or fluorosis. [30]
Although central stenosis is not symptomatic in all people, especially if they have a narrow thecal sac, it may cause bilateral lower extremity pain (radicular pain), with or without a transit pain in the lower extremities that comes on after walking or standing; this phenomenon has been given the name neurogenic claudication.
Neurogenic claudication, which is rarely seen before the sixth decade of life, [84] is a bazaar condition that strikes people after they walk or stand for a certain period of time. Here's the typical scenario: after walking a certain distance, the person begins to experience progressively worse pain, weakness, and heaviness in both lower extremities. These symptoms eventually gets so severe that they are forced to sit down. However, after a short rest, the leg symptomatology miraculously disappears which then allows the person to resume walking until the scenario plays out again. In addition to the sit-down-to-rest fix, the patient may also assume a bent-forward position, such as the one assumed when bending over a shopping cart, which also can promote recovery of the lower extremity symptomatology. In fact, this bent over strolling has been called the "shopping cart sign." [84]
The bilateral radicular pain and neurogenic claudication of central stenosis results from a severe compression of the lumbar nerve roots as they hang in the thecal sac. As we have spoken about before, if a nerve root is compressed with sufficient magnitude and for a sufficient length of time, then its circulatory system breaks down and axons within the nerve root start to die because they can't get enough nutrients and oxygen and can't get rid of their acidic waste products. This axon death causes ectopic discharge, which in turn is perceived as lower extremity pain (and dysfunction if the motor nerves are affected).
The symptoms of neurogenic claudication are thought to be akin to those of radicular pain, only of a more transient nature. Specifically, it is thought that the nerve roots of the cauda equina, which of course are compressed secondary to the stenotic change of the central canal, are just barely getting by with regard to nutrients, oxygen, and waste removal. Since walking increases the use of the nerve roots, there is a temporary increase in the demand for nutrients, oxygen, and waste removal, which cannot been met because of an adequate blood flow through the nerve roots (because of the stenosis). Therefore, after a certain amount of time the axons within the nerve roots become stressed which results in spontaneous firing in the perception of lower extremity pain and temporary dysfunction of the motor roots (which of course connect to the muscles of the legs and make them go haywire). [66,83,84]
The diagnosis and treatment have already been described above. (read more)
Lateral stenosis, is a condition that occurs when either the nerve root canal (nerve root canal stenosis) or the neural foramen (neural foramina stenosis) becomes significantly narrowed secondary to osseous and/or ligamentous thickening, which is typically secondary to degenerative change. A significant narrowing of either of these canals will may result in unilateral radicular pain and not the bilateral radicular pain and/or neurogenic claudication that is seen with central stenosis.
In the neural foramen, quantitatively, the diagnosis is made when either its sagittal height or width become narrowed to a measure less than 15mm and 4mm, respectively. [31] A posterior disc collapse of less than 5 mm will also cause foraminal stenosis. Specifically, research has indicated that foraminal stenosis from a disc collapse occurs at the L5 level, 75% of the time, at the L4 level, 15% of the time, and at the L3 level, 5% of the time. [29]
Anatomically speaking, since the dorsal root ganglia (DRG) typically lives within the neural foramen, compression in this region can be devastating, for this ganglion houses the cell bodies of the sensory neurons which, if compressed, can result in significant pain and disability. Specifically, in an anatomical cadaver study it was learned that in 59% of the cases, the DRG was within the neural foramen. In 33% and 8% of the cases, the DRG was medial to or lateral to the neural foramen, respectively. [31]
Lateral stenosis may also occur within the nerve root canal, which is, confusingly, given many names: lateral spinal canal stenosis, lateral lumbar spinal canal stenosis, lateral canal stenosis, lateral recess stenosis, entrance zone stenosis, mid zone stenosis, foraminal canal stenosis, subarticular stenosis, subpedicular stenosis, and lateral gutter stenosis. [28] I will use the term nerve root canal stenosis, which more accurately describes the condition.
Recall from above sections that the nerve root canal runs from the branch-off point of the thecal sac to the beginning of the neural foramen. Although all lumbar levels have a nerve root canal mid zone, at L5/S1, and sometimes at L4/L5, this nerve root canal also has an entrance zone that is located at the disc-level above its neural foramen exit point. This is because the branch-off point is much higher at the lower two levels when compared to the rest of the lumbar spine.
Disc herniations can cause entrance zone stenosis at these lower levels and superior articular processes hypertrophy (i.e., degenerative changes) can cause stenosis at all levels in all zones. [31]
*Technical point: Disc herniations could "technically" be described as an entrance zone lateral stenosis, or, if severe, central stenosis. However many believe (as do I) that it is inappropriate to "double diagnose" this condition but also calling it stenosis. Therefore, a posterior disc herniation into the traversing nerve root or thecal sac should not be diagnosed as stenosis.
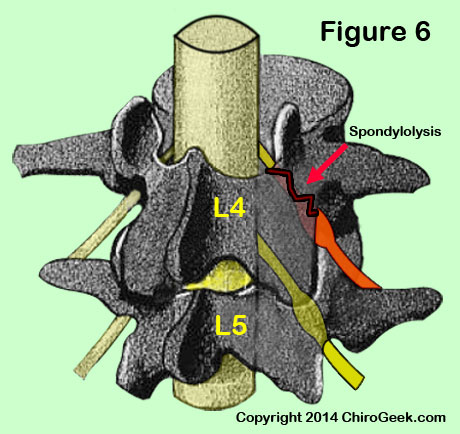 At all levels of the lumbar spine, the nerve root may be compressed as it passes underneath the rough edges of a pars interarticularis (pars) defect (figure 6) or as it rounds the inferolateral pedicle. The former has been called the most common cause of nerve root canal stenosis. [31]
At all levels of the lumbar spine, the nerve root may be compressed as it passes underneath the rough edges of a pars interarticularis (pars) defect (figure 6) or as it rounds the inferolateral pedicle. The former has been called the most common cause of nerve root canal stenosis. [31]
Figure 6 demonstrates a unilateral spondylolysis (black/red jagged line) of the left pars interarticularis that has mechanically irritated the L4 traversing nerve root (orange) as it passes beneath the pars. Such an irritation may cause left lower extremity radicular pain.
Sometimes a bursa irritation at the insertion site of ligamentum flavum, or bone spur formation in the proximal lamina or pars can also intentionally nerve and cause stenosis.
Although compression within the nerve root canal is not symptomatic in all people, [25,95] symptomatic lateral stenosis typically causes unilateral radicular pain, without neurogenic claudication.
Without MRI or CT imaging, is very difficult to differentiate between disc herniation- versus lateral stenosis-related radicular pain. Therefore, in a patient presenting with unilateral radicular pain, the clinician should not assume it is from the disc herniation, for it may be from nerve root canal stenosis or foraminal stenosis.
The diagnosis and treatment of stenosis have already been described above. (read more)
We've already discussed the available treatment options for stenosis quite a bit above (read more). Therefore, I will take this time to talk about some of the classic treatment studies that I have used to support my opinions.
In 2010, Weinstein et al. [4] reported the results of their investigation of 645 patients with confirmed lumbar spinal stenosis (LSS) who were treated at many different centers by many different surgeons. Some patients (n= 289) were randomized into a decompressive surgery group or a nonoperative group. The remaining patients (n=356) were allowed to choose which group they wanted to go in. At the four year follow-up, on an as-treated basis, the patient's who underwent decompression (i.e., laminectomy, laminotomy, discectomy, foraminotomy, medial facetectomy) were doing significantly better (p<0.05) when compared to the patient's who only used nonoperative care. Specifically, the decompression patients had decrease pain, increased function, decreased Oswestry Disability Index scores, and increased patient satisfaction. [4] Other outcome studies have confirm these results. [2] *Comments: as with all studies that attempt to compare conservative care to surgery, a statistical problem always arises when patients "cross over" to the other group, which was an admitted problem with this study as well. Nevertheless, it is still good information.
In 2011, Kovacs et al. [3] reported the results of their systematic review (the highest level of scientific evidence {if done correctly}) that pooled the results from five very high quality randomized control trials (RCTs) (out of 739 mediocre quality RCTs) in order to compare the outcomes of spinal surgery (i.e., a mix of fusion, interspinousprocess device, and decompression) versus conservative care for patients suffering radicular pain secondary to stenosis. At the minimal two-year follow-up (range: 2–10 years), the patients who had undergone surgery were statistically (p<0.05) more improved when compared to patients who did not. Specifically, the surgery group demonstrated decreased pain, decreased disability, and increased quality of life. However, surgery was not superior for improving patient walking distance and the beneficial effects tended to "decrease with time but still remain significant till 4 (and possibly 10) years later. [3] *Comments: one of the five RCTs (the interspinous process device study) was sponsored by the industry and one of the authors had admitted conflicts of interest. Whether or not those biases affected the study result is unknown.
In 2011, Radcliff et al. [23] reported the results of their study that looked at the timing of surgery for symptomatic stenosis. Specifically, they compared the surgical outcomes (90% of the surgeries were decompressions) from two groups of patients: the first group (n=405) had suffered the symptoms of stenosis (i.e., leg pain greater than low back pain) for less than 12 months and the second group (n=229) had suffered symptoms for greater than 12 months. At the four-month follow-up, the patients with symptoms less than 12 months significantly (p<0.05) outperformed those with symptoms of a duration greater than 12 months with regard to improvement in pain and function. Furthermore, patients with longer symptom duration necessitated significantly more additional surgeries at both the two and four year outcomes. Interestingly, stenosis patients with concomitant degenerative spondylolisthesis demonstrated no difference between the groups. [23] The take-home message seems to be this: if your stenosis is not caused by degenerative spondylolisthesis, then you should not wait too long before having that recommended surgery, for if you do, you may screw up your chances for having an excellent result.
1) Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management for lumbar spinal stenosis: 8 to 10 year results from the Maine Lumbar Spine Study. Spine 2005; 30:936-943.
2) Amundsen T, Weber H, Nordal HJ, et al. Lumbar spinal stenoosis: conservative or surgical management?A prospective 10-year study. Spine 2000; 25:1424-1436.
3) Kovacs FM, Urruita G, Alarcon JD. Surgery versus conservative treatment for symptomatic lumbar spinal stenosis. A systematic review of randomized controlled trials. Spine 2011; 36:E1335-E1351.
4) Weinstein JN, Tosteson TD, Lure JD, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the spine patient outcome research trial. Spine 2010;35:1329-1338.
5) Herkowitz HN, Dvorak K, Bell G, et al (2004). The Lumbar Spine (3rd ed.). Philadelphia, PA: Lippincott Williams & Wilkins.
6) Modic MT, Masaryk TJ, Ross JS (1989). Magnetic resonance imaging of the spine. Chicago, IL: Year Book Medical Publishers.
7) Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence and association with symptoms: The Framingham study. Spine J 2009;9:545-550.
8) Bolender NF, et al. Role of computed tomography and myelogram in the diagnosis of central spinal stenoosis. J Bone Joint Surg Am. 1985; 67:240-246.
9) Sortland O, et al. Functional myelography with metrizamide in the diagnosis of lumbar spinal stenosis. Acta Radiol Suppl 1977;355;42-54.
10) Verbiest H. Pathomorphologic aspects of developmental lumbar stenosis. Orthop Clin North Am 1975;6:177-196.
11) Wiltse LL. History of spinal disorders. In: Frymoyer JW, editor. Adult spine. New York: Raven Press; 1991. p.33-35.
12) VanGelderen C. Ein Orthotisches (lordotisches) Kaudasyndrom. Acta Psychiatr Neurol 1948; 23:57-68.
13) Verbiest H. The radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surgery Br 1954; 36:230-237.
14) Bae HW, Rajaee SS, Kanim LE. Nationwide trends in the surgical management of lumbar spinal stenosis. Spine 2013;38:916-926.
15) Deyo RA. Treatment of lumbar spinal stenosis: a balancing act. Spine J 2010;10:625-627.
16) de Schepper EIT, Overdevest GM, Suri P, et al. Diagnosis of lumbar spinal stenosis: An updated systematic review of the accuracy of diagnostic tests. Spine 2013; 38:E469-E481.
17) Genevay S, Atlas SJ. Lumbar spinal stenosis. Best Pract Res Clin Rheumatol 2010;24:253-265.
18) Steurer J, Roner S, Gnant R, et al. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Musculoskelet Disord 2011;12:175.
19) Suri P, Rainville J, Kalichman L, Katz JN. Does this older adult with lower extremity pain have the clinical syndrome of lumbar spinal stenosis? JAMA 2010;304:2628-2636.
20) Katz JN, Harris MB. Clinical practice: lumbar spinal stenosis . N Engl J Med 2008;358:818-825.
21) Forsth P, et al. Does fusion improve the outcome after decompressive surgery for lumbar spinal stenosis? A two-year follow-up study involving 5390 patients. Bone Joint J 2013; 95-B:960-965.
22) Aalto TJ, Malmivaara A, Kovacs F, et al. Preoperative predictors for postoperative clinical outcome in lumbar spinal stenosis. Systematic review. Spine 2006;31:E648-E663.
23) Radcliff KE, Rihn J, Hilibrand A, et al. Does the duration of symptoms in patients with spinal stenosis and degenerative spondylolisthesis affect outcomes? Analysis of the Spine Outcomes Research Trial. Spine 2011; 36:2197-2210.
24) Skolasky RL, Maggard AM, Thorpe RJ, et al. United States hospital admissions for lumbar spinal stenosis; racial and ethnic differences, 2000 through 2009. Spine 2013; 38:2272-2278.
25) Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. N Engl J Med 1994;331:69-73.
26) Jenis LG, An HS. Spine update: lumbar foraminal stenosis. Spine 2000;25:389-394.
27) Burton R, et al. Causes of failure of surgery on the lumbar spine. Clin Orthop 1981;157:191-197.
28) Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine 1988;13:313-320.
29) Hasegawa T, et al. Lumbar foraminal stenosis: Critical heights of the interverteral discs and foramina. J Bone Joint Surg Am 1995;77:32-38.
30) Arnoldi CC, et al. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop 1976;115:4-5.
31) Botwin KP, Gruber RD. Lumbar spinal stenosis: anatomy and pathogenesis. Phys Med Rehabil Clin N Am 2003;14:1-15.
32) Yabuki S, Fukumori N, Takegami M, et al. Prevalence of lumbar spinal stenosis, using diagnostic support tool, and correlated factors in Japan: a population-based study. J Orthop Sci 2013; 18:893-900.
33) Chen E, et al. Surgical treatment patterns among Medicaid beneficiaries newly diagnosed with lumbar spinal stenosis. Spine J 2010;10:588-594.
34) Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med 2008; 358:818-825.
35) Deyo RA. Treatment of lumbar spinal stenosis: a balancing act. Spine J 2010; 10:625-627.
36) Crock H. Normal, pathologic anatomy of the lumbar spinal nerve root canals. J Bone Joint Surg [Br] 1981; 63:487-490.
37) Postacchini F, Gumina S, Cinotti G, et al. Ligamentum flavum in lumbar disc herniation and stenosis: light and electron microscopic morphology. Spine 1994;19:917-922.
38) Bose K, Balasubramaniam P. Nerve root canals of the lumbar spine. Spine 1984;9:16-18.
39) Rauschning W. Normal and pathologic anatomy of the lumbar root canals. Spine 1987;12:1008-1019.
40) Wu et al. interspinous spacer versus traditional decompressive surgery for lumbar spinal stenosis: a systematic review and meta-analysis. PloS ONE 2014; 9:e97142.
62) Comer CM, et al. Assessment and management of neurogenic claudication associated with lumbar spinal stenosis in a UK primary care musculoskeletal service: a survey of current practice among physiotherapists. BMC Musculoskelet Disord 2009;10:121.
66) Ooi Y, et al. Myeloscopic study on lumbar spinal canal stenosis with special reference to intermittent claudication. Spine 1990;15:544-549.
75) Albert HB, Briggs AM, Kent P, et al. The prevalence of MRI-defined spinal pathoanatomies and their association with Modic changes in individuals seeking care for low back pain. Eur Spine J 2011;20:1355-1362.
83) Ikawa M, Atsuta Y Tsunekawa H. Ectopic firing due to artificial venous stasis in rat lumbar spine canal stenosis model: a possible pathogenesis of neurogenic intermittent claudication. Spine 2005;30:2393-2397.
84) Porter RW. Spinal stenosis and neurogenic claudication. Spine 1996; 21:2046-2052.
95) Jarvik JG, Hollingworth W, Heagerty PJ, et al. Three-year incidence of low back pain in an initially asymptomatic cohort: Clinical and imaging risk factors. Spine 2005;30:1541-1548.