The Lumbar Fusion Page |
 |
Lumbar fusion is a very invasive (destructive) type of spine surgery that is performed in over 400,000 Americans each year because of intolerable low back pain, [4,39] and its frequency of use is skyrocketing. In a 2012 publication, Rajaee et al. reported a 137% increase in the utilization of lumbar fusion between 1998-2008, which was higher then either hip or knee replacement surgery. [39]
 In addition to this significant rise in utilization, the cost of a single fusion surgery has reached an astonishing $81,960, which represents a 3.3 fold increase since 1998, [39] and the yearly price tag for all fusions performed in the US, as last calculated in 2008, was a staggering $33.9 billion. [39]
In addition to this significant rise in utilization, the cost of a single fusion surgery has reached an astonishing $81,960, which represents a 3.3 fold increase since 1998, [39] and the yearly price tag for all fusions performed in the US, as last calculated in 2008, was a staggering $33.9 billion. [39]
Figure left represents a modern interbody fusion in which the pain-generating disc has been replaced by a cage (interbody spacer) that is surrounded by bone chips (or other fusion material), which are usually taken from the patient's own hip. Bone chips (bone graft material) are also packed between the especially-prepared transverse processes and/or between the facet/lamina region of the two vertebra. Posterior instrumentation, i.e., rods and screws, are also inserted and cinched down in order to place the motion segment in the proper sagittal alignment (slight extension) and, like a cast, will hold the motion segment very still while the bone chips to "ossify" or "harden."
Notwithstanding its financial strain on the US economy, the efficacy (safety and effectiveness) of lumbar fusion remains equivocal (debatable) as demonstrated by the recent publication of two meta-analyses (a special type of study that puts forth the highest level {level I} of scientific evidence) that have failed to demonstrate fusions superiority to simple (and much less costly) nonoperative care, such as physiotherapy and exercise. [40,41]
On the other hand, there is still the Volvo Award-Winning study of Fritzell et al. [48] which was a multi-center randomized-control trial (which is also level I evidence) that demonstrated lumbar fusion significantly outperformed nonoperative care with regard to pain decrease, functional improvement, satisfaction, and return to work. [48] Then there was the other famous multi-center randomized-control trial (the SPORT study) by Weinstein et al. [53] that concluded spondylolisthesis and stenosis patients achieve superior fusion outcomes (how good the patient thinks he or she did after the fusion) when compared to nonoperative care and held that improvement until the end of the study, which was four years. [53]
So what should a person believe? The meta-analysis or very well-done randomized control trial? First of all let me say that these level I meta-analyses always seem to come up negative with regard to any type of treatment perhaps because it's just not right to compare apples with oranges. A meta-analysis puts forth its opinions by pooling the data from many different papers on the subject, many of which used subtle differences in surgical technique, data collection methodology, and different outcome questionnaires. Therefore, I just don't believe that they are as powerful as a well designed multicenter randomized-controlled trial and should be taken with a grain of salt. I'm not the only one to feel this way. Check out the quote from Penta et al. [54] with regard to what type of study should be used to verify efficacy of the procedure:
"The only accurate way of assessing the role of lumbar fusion in the treatment of low back pain is by prospective, randomized, controlled, long-term studies comparing spinal fusion with nonoperative treatment."
There is no question that spinal fusion has its rightful place amongst the arsenal of weapons that we have to treat chronic intractable spine pain; however, its must be used judiciously and performed in just the right patient by just the right surgeon. *In fact, choosing a spine surgeon to do the fusion is most likely more important than the actual technique that he or she uses. I can't stress this point enough; you have got to do your homework when selecting a surgeon.
Although lumbar fusion should never be used for a run-of-the-mill paracentral disc herniation, it has been found efficacious (safe & effective) for the treatment of scoliosis, spondylolysis, spondylolisthesis, stenosis, internal disc disruption, isolated disc reabsorption, facet syndrome, vertebral endplate syndrome, recurrent disc herniation, and giant disc herniation, as well as for fracture, tumor and infection of the spine.
The first fusion was performed in 1911 by Albee et al. [31] who used bone from the tibia (leg bone) in order to perform a crude posterolateral fusion (PLF) for a patient suffering painful tuberculosis of the spine. [31] The technique then underwent several important modifications (especially by the addition of posterior instrumentation) over the years until the modern version of PLF came to being as reported by Wiltse in 1975. [32] (We shall talk more about the techniques farther below.)
The first interbody fusion was described by Cloward in the early 50s [44,45] and was used as an alternative to simple discectomy, which was having miserable results at the time. Instead of a metal alloy cage, a fashioned bony plug was inserted for a posterior approach and acted as the interbody spacer; posterior instrumentation was not used. This technique was named posterior lumbar interbody fusion (PLIF) and is still used today; however, it is associated with higher post-fusion morbidity (it tends to hurt patients more) and has really fallen out of favor because of the magnitude of bony destruction necessitated and the long nerve root retraction times.
Posterior lumbar interbody fusion soon evolved into a variety of other interbody fusion techniques ("interbody" means that the center of the disc was removed and a sturdy replacement {usually a cage} was installed) that include anterior lumbar interbody fusion (ALIF), transforaminal lumbar interbody fusion (TLIF) and 360° fusion, all of which will be discussed farther on down the page. There are other even more recent fusion techniques which are still considered experimental and will not be discussed here.
All of these fusion techniques can be performed via "minimally invasive surgery" (MIS), which again will not be discussed here, for they are still considered experimental in my book.
Recovering from fusion (especially interbody fusion) is typically not a piece of cake, for during the procedure a significant amount of tissue is destroyed and injured, which takes time to heal. Plan on being out of work for at least 2-3 months and even longer if your job is arduous. Another important fusion fact is that you will (or at least should) have permanent limitations with regard to heavy lifting; repeated bending, twisting, and stooping at the waist; and participating in contact sports or similarly physical activity. Without these limitations, you may very well end up needing revision surgery in the not so distant future.
Unfortunately, it is not uncommon for complications to occur during and after the fusion procedure. Such complications include dural tear, vertebra fracture, nerve root injury, bone/disc infection, seroma/hematoma formation, wound infection/dehiscence, blood clot/pulmonary embolism, pseudoarthrosis (failure of the motion segment to fuse together), hardware irritation/failure, cage displacement and adjacent-segment disease (ASD), which may or may not require revision (second) surgery.
If recombinant human bone morphogenetic protein-2 (rH-BMP-2) was used during the procedure, then the additional complications of vertebral body destruction (osteolysis), excessive bone growth into the spinal canal (heterotopic bone growth), and even the possible development of cancer; [43] however, the jury (no pun intended) is still out on this possible complication as other studies have shown no relationship between rH-BMP-2 and cancer. [52]
Although rates of revision surgery after fusion can be surprisingly high, [14] for patients who are an ideal candidate and have found a spine surgeon with an outstanding record of fusion success, the clinical outcomes (how good the patient thinks the surgery did) and patient-satisfaction can be quite high.
A motion segment (i.e., a vertebra-disc-vertebra complex) typically generates chronic back pain when inflamed nociceptive nerve fiber (pain-carrying nerves) within the disc, facets, endplates, and/or fractured pars interarticulari becomes mechanically irritated from the natural movement of the motion segment which results from the activities of daily living (i.e., getting out of bed, putting on clothing, preparing meals, going shopping etc.). In other words, when the motion segment moves, it hurts.
 Therefore, as the theory goes, in order to stop the pain, you must permanently stop the movement of the motion segment, which is accomplished by a spine surgery called fusion.
Therefore, as the theory goes, in order to stop the pain, you must permanently stop the movement of the motion segment, which is accomplished by a spine surgery called fusion.
In the simplest of terms, this surgery "fuses" (welds, glues or ossifies) the two vertebra of the motion segment into one solid unit, thereby eliminating the pain-generating movement.
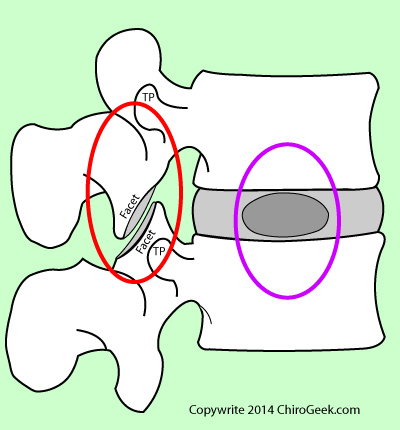
Figure left is a sagittal view (from the side) of a typical lumbar motion segment. In order to stop all movement and eliminate pain, the motion segment is fused in the posterior column (red circle) by packing bone chips (often taken from the patient's own hip) into the spaces between the transverse processes (TP) and prepared (decorticated) facet/lamina region. With the passage of time, these bone chips will grow into one mass and "glue" the posterior column of the motion segment firmly together. Often times, in addition to the posterior column fusion, the nucleus and part of the annulus will be removed and replaced by a spacer (cage) which is surrounded by bone chips. Again, with the passage of time the bone chips grow into one mass and unite the two vertebral bodies. (See image left "fused.")
If only the posterior column is fused, then the procedure is called a posterolateral fusion. (PLF) If the anterior column is fused as well, then the procedure is called an interbody fusion.
The goal of every fusion surgery is to achieve a solid ossification (solid fusion or arthrodesis) between the two vertebra of the motion segment, for recent medical research has demonstrated that patients who are successfully fused, have significantly better clinical outcomes. [35]
 Unfortunately not only is fusion the most invasive (destructive) type of spine surgery, it also has one of the highest rates of failure, which has been calculated at 13% in a very large study of 1,680 patients with five years of follow-up. [14]
Unfortunately not only is fusion the most invasive (destructive) type of spine surgery, it also has one of the highest rates of failure, which has been calculated at 13% in a very large study of 1,680 patients with five years of follow-up. [14]
Figure 1 is a cartoon depiction of the posterolateral fusion (PLF) procedure, which has been completed only on the left. In reality it's done on both sides and used to eliminate all pain-producing motion in the posterior column of the motion segment. Although it might be an effective treatment intervention for conditions of instability (i.e. spondylolytic spondylolisthesis and fracture), PLF does not eliminate all motion in the anterior column (which is where the disc is) and therefore is a poor choice for treating discogenic pain.
In other words, you could say that at least 1 out of every 10 patients will be forced to go through a second spine surgery within five years from the date of the first spine surgery because of recurrent pain. [14]
Anecdotally, the rate of failed fusion (as defined by poor patient satisfaction after the fusion) has been placed at 33%! *Don't stop reading, however, for fusion certainly can be a real lifesaver if you are the right candidate, and I know of many fusion success stories.
One of the reasons for the high rate of fusion revision surgery is because of a well-known phenomenon called the "domino affect." The domino effect, which is more properly called adjacent segment pathology (ASP) or adjacent segment degeneration (ASD), is said to have occurred if either the motion segment above or below the original site of fusion develops a premature and painful degenerative process, which in turn may necessitate revision surgery. Interestingly, it's almost always (90% of the time in one study) the motion segment above the level of fusion that becomes painful and necessitates revision surgery after fusion. [17,18]
What causes ASP? When one of the five motion segments of the lumbar spine is fused (*especially if the surgeon fails to fuse the motion segment with sufficient lordosis), there is a disruption of the axial load and motion sharing that naturally occurs equally between all segments. However, when one motion segment is fused, the segment above (and to a lesser extent below) has to do double-duty with regard to handling trunk movement and axial load, which in turn may cause rapid degenerative disease and lead to a new source of pain. [18] Although, a failure of the adjacent disc (i.e., it develops painful annular tears and/or herniations) is usually the cause for second surgery, the development of a painful facet syndrome or stenosis may also occur.
What's the quickest way to start a brawl in a room full of spinal surgeons? Ask them "what is the best fusion technique for the treatment of chronic discogenic low back pain?" Many spine surgeons are strongly opinionated when it comes to their craft and rarely can agree (sometimes notwithstanding quality research) which technique is best for treating a specific diagnosis—their way is always the best way.
So which one is the best? That really depends on the patient's unique situation, and I would be happy to discuss this with you privately through one of my Coaching Sessions.
Let's talk about the most common fusion techniques.
 Of all the classic fusion techniques, PLF is the least invasive, for it does not require entering the epidural space and therefore eliminates the possibility of lumbar nerve root and/or thecal sac injury. {and once nerve roots are damaged via during interbody fusion, they often go on to cause life-long chronic lower extremity pain and/or radiculopathy.}
Of all the classic fusion techniques, PLF is the least invasive, for it does not require entering the epidural space and therefore eliminates the possibility of lumbar nerve root and/or thecal sac injury. {and once nerve roots are damaged via during interbody fusion, they often go on to cause life-long chronic lower extremity pain and/or radiculopathy.}
Posterolateral fusion is the grandfather of all fusion techniques and was developed just over 100 years ago by Albee et al. [31] Since then, PLF has come a long way and is still used by surgeons to correct posterior column problems, such as spondylolysis and spondylolisthesis. It is also used to correct structural deformities such as scoliosis.
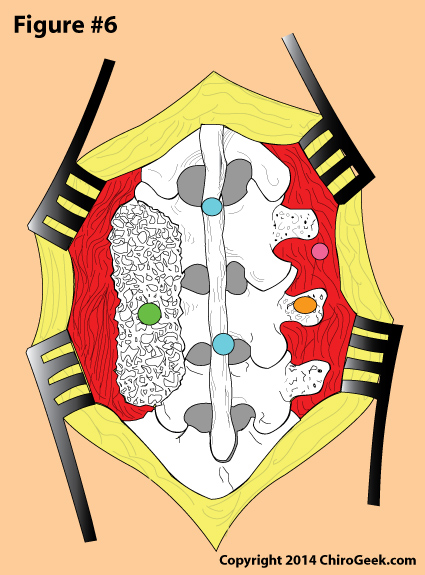
Figure 6 is a from-behind view (PA view) of a lumbar spine that is opened up (the skin, fascia, and muscles are dissected and pulled away from the lumbar spine) for the PLF procedure. Note that bone chips have been packed between the decorticated transverse processes, facets, and lamina on the right at L3/4 and L4/5 (green dot). The surgeon, who has just finished decorticating (roughing up) the right transverse processes (orange dot), is now about to pack bone chips on the left side to complete the surgery. Posterior instrumentation was not used this time.
One of the most common conditions treated by PLF is isthmic or degenerative spondylolisthesis where it can be used as a stand-alone procedure or supplemented with segmental pedicle screw fixation. What does that mean? It can be either used alone or you can use it in combination with pedicle screws and rods which span the motion segment that you are treating. This instrumentation not only acts as a "cast" to encourage solid fusion, but also add strength to the fusion construct.
Posterolateral fusion is also commonly used as an augmentation (supplementation, then edition to) to all four of today's major fusion techniques: TLIF, ALIF, XLIF and PLIF.
In the mid-1940s, Dr. Ralph Cloward [44,45] developed a novel (new) type of fusion for the treatment of disc herniation. The impetus for this innovative surgical technique was the poor results that he was achieving with the discectomy procedure of the time—70% of these patients had poor outcomes. Therefore, he stopped using discectomy and over an eight-year period performed 321 consecutive fusions using a technique that he called posterior lumbar interbody fusion (PLIF). The results of this history-making experiment, which were reported in 1952, demonstrated that 85% of the patients achieved a very satisfactory outcome. [45]
*Anecdotally, the most likely explanation for Cloward's poor discectomy results was because back in those days they didn't understand that certain conditions of the disc (central disc herniation, small contain disc herniation, disc bulge, recurrent disc herniation, isolated disc reabsorption, and internal disc disruption) respond very poorly to discectomy and such as surgery shouldn't have been performed in the first place.
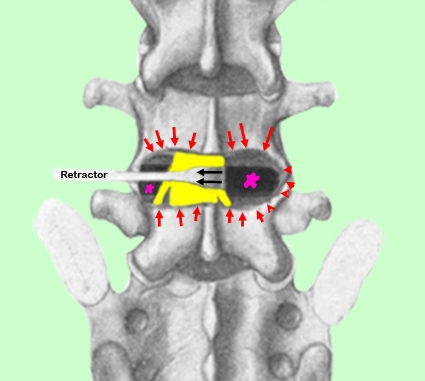 Figure left demonstrates how Cloward's PLIF technique was performed, which is quite similar to how it is performed today: first, as noted in figure left, a wide laminotomy is carried out (red arrows) which, destructively, creates a large window at the disc level. In other words, a hole is cut in the posterior portion of the lumbar spine in order to again access to the disc space.
Figure left demonstrates how Cloward's PLIF technique was performed, which is quite similar to how it is performed today: first, as noted in figure left, a wide laminotomy is carried out (red arrows) which, destructively, creates a large window at the disc level. In other words, a hole is cut in the posterior portion of the lumbar spine in order to again access to the disc space.
Next the traversing nerve root and the thecal sac are retracted out of the way (black arrows) in order to expose the pain-generating disc. Then the entire half of the disc is removed, except for the anterior and the lateral annulus fibrosus, which leaves a hollow space (large pink star).
Next, while retraction of the neural structures remains in effect, bone plugs (we use metal alloy cages or spacers in this day and age) are jammed into the disc space which will eventually ossify and unite (fuse) the two vertebral bodies of the motion segment into one unit (not shown).
Next, the same procedure will be carried out on the opposite side: the side opposite traversing nerve root and thecal sac will be tractioned out of the way, the disc tissue will be removed, and bony plugs will be jammed in to the disc space.
 Finally, bone chips (bone graft material), which have the capability to ossify together like glue, will be used to fill in the wide laminotomy in order to fuse (weld together) the posterior column of the motion segment into one unit. [44,45] (Figure left)
Finally, bone chips (bone graft material), which have the capability to ossify together like glue, will be used to fill in the wide laminotomy in order to fuse (weld together) the posterior column of the motion segment into one unit. [44,45] (Figure left)
In addition to Cloward's procedure, in this day and age, posterior instrumentation (pedicle screws and rods) is employed on each side in order to stabilize the motion segment while ossification occurs and to asssure that the vertebrae are in sagittal alignmennt (slight extension). This instrumentation could later be removed.
Although PLIF was a very effective intervention in its day, it has really fallen out of favor and given way to some of the less invasive interbody fusion techniques, such as TLIF and ALIF, which do not require such prolonged retraction of the neural structures (ALIF does not require any retraction, for it does not even enter the epidural space). Furthermore, because of the potential for spinal cord damage, PLIF is limited to the L3-S1 levels. In other words, if you have a L1 or L2 problem that needs fusion, PLIF cannot be performed. [51]
Such traction techniques have been associated with permanent nerve root damage and resultant chronic radicular pain. In fact in one 2002 study, 13.6% of the patients treated with one PLIF technique ended up suffering permanent iatrogenic (from the procedure itself) nerve root damage with resultant chronic radicular pain at follow-up! [47]
Let's move on to the more popular techniques.
One of the more popular interbody fusion techniques is called transforaminal lumbar interbody fusion (TLIF), which was first described by Harms and Jeszenszky in 1998. [7]
Unlike PLIF, which destroys much of the posterior arch (both facets, laminae and spinous process) and requires prolonged neural structure retraction on both sides, TLIF is much less destructive and requires only minimal (if any) unilateral (only one side) retraction. [8,9] Undoubtedly because of the minimized nerve root retraction, medical research has demonstrated that the TLIF technique results in less iatrogenic nerve root damage (i.e., postoperative radiculitis) as compared to PLIF. [49]
In a nutshell, here's how the technique works: after the skin, fascia, and muscle posterior to the motion segment is dissected and retracted away (just like in the PLF and PLIF procedures), the pain-generating disc is approached posterolaterally in order to enter the epidural space through the facet joint (which is usually completely removed {red, figure below).
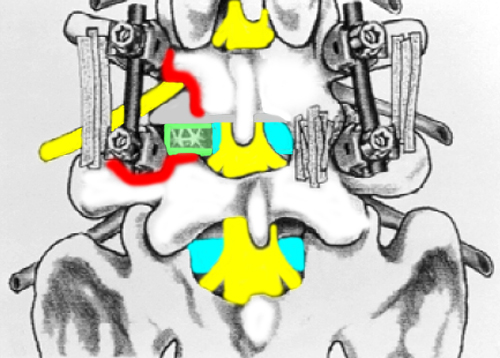 This approach puts the surgeon into the posterolateral region of the epidural space which is considered a safe-zone called Kambin's triangle. The reason it is called the safe-zone, is because it is right in between the exiting and traversing nerve roots.
This approach puts the surgeon into the posterolateral region of the epidural space which is considered a safe-zone called Kambin's triangle. The reason it is called the safe-zone, is because it is right in between the exiting and traversing nerve roots.
Figure left is a posterior to anterior view of the L4/5 motion segment that represents a typical transforaminal lumbar interbody fusion with PLF augmentation and instrumentation. Note how the left facet (area between red) has been removed and a cage (green) has been inserted through the posterolateral disc via the annulotomy, into the disc space. Note the posterior instrumentation (pedicle screws and rods) as well as the strips of bone which represents the PLF.
Therefore, very little retraction of either of the nerve roots will be necessary; although, they will have to be at least protected by the retractor, so some nerve root contact is inevitable, but not nearly as much as with TLIF.
Next, using special tools the surgeon will create a box-shaped hole in the posterolateral annulus fibrosus of the disc, through which all the work will be done. Next, as much of the nucleus pulposus as possible will be removed and then the endplates will be will be scraped away with just enough force to create a tiny amount of bleeding of the subchondral bone. It is important that the surgeon doesn't scrape away too much bone, for this could weaken the subchondral bone and allow the cage to collapse or sink into the vertebrae postoperatively, which is a complication called subsidence.
After the disc space is prepared, an interbody graft (cage) (green, figure above), which is typically made of polyetheretherketone (PEEK) will be inserted and then bone chips will be packed all around it in order to fuse the vertebral bodies of the motion segment together.
Next, very carefully (some surgeons use Stealth technology to guide them) pedicle screws will be inserted above and below in both vertebra of the motion segment and rods will be attached to those. The rods can then be cinched down in order to establish the proper sagittal alignment (extension) of the motion segment.
The pitfalls of this technique are (1) there is a steep learning curve and (2) the workspace for the surgeon is limited compared to traditional PLIF, which removes both lamina and the spinous process.
Unfortunately, and perhaps surprisingly, to date there continues to be not a single randomized control trial (level I evidence) that compares TLIF to either PLIF or ALIF. (see my Medline search)
Hackenberg's 2005 prospective investigation on the efficacy of TLIF, [51] is the one commonly reported, so let's review that quickly. Hackenberg prospectively treated 54 patients with instrumented TLIF, without rH-BMP-2 augmentation, with the goal of studying their long-term outcomes. 93% of the patients were available for a three year assessment which revealed a significant decrease in pain, increase inability to function, and an 89% rate of solid fusion. There was, however, an 8% serious complication rate that included one case of infection (all the instrumentation had to be removed in the fusion had to be repeated from the entry approach); one case of unfixable radiculopathy (the surgery created a new sciatica); and one contralateral herniation, which was thought to have occurred because not enough of the nuclear material was removed during the surgery. [51]
All in all, TLIF, which is being performed with minimally invasive techniques these days {we can talk about that during a coaching session if you want, but I won't discuss it here}, is probably my favorite fusion technique as it avoids excessive manipulation of the nerve roots and is more minimally invasive than ALIF or PLIF.
Anterior lumbar interbody fusion, or ALIF, is an interbody fusion procedure that accomplishes fusion between the vertebral bodies of the motion segment by approaching them from the front (i.e., through the belly).
Capener first introduced a crude anterior approach for the treatment of 32 patients with a spondylolisthesis way back in 1932 [Phan(2016) 4]. In 1948, Lane and Moore first used the ALIF procedure for the treatment of lumbar degenerative disc disease [Phan 33, 34]. The technique became more popular in the 1970s and 80s but the success of solid interbody fusion is not that great. Part of the reason is there is no local bone available to use as graft material.
With the invent of of lumbar-tapered cage systems, the success of fusion significantly increased (we still use these today). Benefits of this trapezoid -shaped cage include improved lumbar sagittal lordosis, better reaming of the endplates, and the ability to load the cage with rhBMP-2, which is allowed by the FDA. However, these titanium cages are "harder" then bone (have a higher Young's modulus of elasticity) and therefore greatly increase the chance of subsidence (the cage sinks into the vertebral endplates during the inflammatory process of fusion). Furthermore, the titanium produces MRI and CT artifact which make it difficult to visualize the fusion constrict.
 Polyether ether ketone (PEEK) were next introduced by AcroMed in the 1990s which significantly solved the subsidence problem and are much more imaging friendly. They have also been found to be resistant to microbial adhesion and therefore decrease the rates of infection following surgery. One limitation to the use of PEEK is that it does not integrate into the native vertebral endplates well. In other words, it is not super bioactive, in and of itself. Numerous augmentations have been developed to improve this bioactivity failure. The best-known would be recombinant human bone morphogenetic protein-2 (rhBMP-2). Some newer cage designs include a combination of PEEK with added titanium or hydroxyapatite.
Polyether ether ketone (PEEK) were next introduced by AcroMed in the 1990s which significantly solved the subsidence problem and are much more imaging friendly. They have also been found to be resistant to microbial adhesion and therefore decrease the rates of infection following surgery. One limitation to the use of PEEK is that it does not integrate into the native vertebral endplates well. In other words, it is not super bioactive, in and of itself. Numerous augmentations have been developed to improve this bioactivity failure. The best-known would be recombinant human bone morphogenetic protein-2 (rhBMP-2). Some newer cage designs include a combination of PEEK with added titanium or hydroxyapatite.
IMAGE LEFT: this is a hybrid PEEK + Titanium cage which may increase the chances of solid interbody fusion. The PEEK component will resist subsidence, yet the titanium component will increase its bio-integration into the native bone. Specifically, the PEEK cage is impregnated with particles of titanium oxide during the manufacturing process , which has shown superiority over PEEK alone. [101, 102 {Phan- 2016}]
Although such an approach spares contact with the delicate posterior neural structures (i.e., the thecal sac, nerve roots, spinal nerve and dorsal root ganglia) and eliminates any posterior muscle damage that is associated with the posterior fusion procedures, ALIF is associated with male sterility via a condition called retrograde ejaculation secondary to damage to the hypogastric plexus from the surgical approach.
ALIF can be performed in one of three ways: via an open, mini-open or laparoscopic approach. Research has not generally demonstrated that one technique is superior to the other. Although some of these approaches may be more amenable to treat certain conditions, the most important thing is that the surgeon uses the approach he or she is more comfortable with.
Because the ALIF procedure is unable to reach most of the epidural space, it is not effective for the treatment of central or lateral stenosis. It is better designed for the treatment of disc-related problems (isolated disc reabsorption, internal disc disruption, or reoccurrence disc herniation), kyphotic deformities, and/or spondylolisthesis.
Anterior lumbar interbody fusion is also an option for treating patients who had suffered a failed posterior fusion procedure. In these patients, you don't want to go through the epidural space again in order to correct a pseudoarthrosis (failure to fuse). Therefore, it's best to come from the anterior in order to fixed the problem.
Another big limitation stems from the fact that ALIF can only be used for problems at the L4 and L5 motion segment. This is because the inferior vena cava and aorta block the anterior disc space above the L4 level.
Besided complications of pseudoarthrosis, vertebral body fracture, and all the other complications mentioned for the other fusion procedures, ALIF is the only fusion procedure that could make the male patient infertile after the procedure, via a condition called retrograde ejaculation.
The hypogastric plexus, which contains the nerves to the bladder and urethra, is in very close proximity to the anterior portion of the L4 and L5 discs. Although we are still not sure of the mechanism, between 0.4% and 6% of men will lose the ability to ejaculate sperm from the penis (the sperm harmlessly goes into the bladder instead) after the ALIF procedure, and become completely infertile. [56-58]
Of the two approaches that can be used to complete ALIF, is the transperitoneal approach that has a higher chance of causing male sterility. More specifically, medical research has demonstrated that there is a 10 times greater chance of suffering retrograde ejaculation with the transperitoneal approach, when compared to the retroperitoneal approach. [58]
Quality randomized-control trials comparing ALIF to either TLIF or PLIF did not exist at the time of this writing (May, 2014). Therefore, we must use lesser quality evidence to establish efficacy. In other words, we have to use a retrospective study to see if this type of fusion actually works and is safe.
In 1997, Penta and Fraser [54] reported the results of their retrospective study which followed a group of 125 patients who were treated with ALIF, mainly for the diagnosis of discogenic pain. The beauty of this rare study was that these patients were followed for 10 years and that 82% of them were still available to answer questions at that time point. Although 78% of the patients reported either complete or good relief from their chronic back and/or leg pain, only 34% had a good or excellent return of function. In other words, the pain was no longer a problem for most of them; however, they were unable to function at a very high level with regard to activities such as moderate to heavy work, sports, or other similar activities. [54] Do these findings remind you of something you just read? These findings were actually quite similar to the very first outcome study ever performed on ALIF, which was done almost 70 years ago! [55]
To Make up for some of the inherent weakness of stand-alone ALIF, surgeons will commonly add the PLF fusion procedure to the ALIF procedure, which results in a technique called 360° Fusion. [36,37]
Now, the procedure can address common conditions such as spondylolisthesis and stenosis; however, 360° fusion requires much more time on the operating table or requires coming back to the operating room a few days after the ALIF is completed.
Although I know spine surgeons who swear by the procedure, the research doesn't support it very well. For example, in 2002 Christensen et al. reported the results of their randomized control trial that compared instrumented PLF against 360° fusion for the treatment of degenerative spondylolisthesis. At the two-year follow-up, there is no difference between the patient outcomes as both groups of patients improved statistically the same—78% patient satisfaction was reported in both groups. [36] The same groups of patients were followed an additional three years which revealed similar results: there was no difference in outcomes between the two groups. However, The 360° Fusion patients did have a higher rate of successful interbody fusion and needed fewer reoperations. [37]
Lateral lumbar interbody fusion (LLIF), also known as extreme lateral lumbar interbody fusion (XLIF) or sometimes just a "retroperitoneal transpsoas approach.
LLIF is a minimally invasive surgical procedure that allows surgical access to the lateral intervertebral disc of L1, L2, L3, and (although challenging) L4.
It was first described by Obenchain in 1991 but has only become more mainstream in the last decade. It was hoped that LLIF would become the new gold standard of interbody fusion by eliminating the well-known surgical complications of ALIF (i.e., retrograde ejaculation and great vessel injury) and PLIF/TLIF (i.e., exiting and traversing nerve root injury).
Although LLIF has in fact eliminated the aforementioned surgical complications, it has created a new one: lumbar plexus injury!
Let me explain. In order to gain access to the lateral portion of the disc, the surgeon must "tunnel" through the psoas major muscle. And as any first year medical/chiropractic/physical therapy student knows, the ventral rami of the lumbar plexus travel right through the center of this muscle and are therefore subject to serious injury by the surgical dissection.
Although neuromonitoring is used and the surgeon is being as careful as possible, these nerves frequently get damaged by instrument trauma or overstretching of the nerve during dilation of the passageway.
Let's take a look at a couple of studies which support my above verbiage:
Cummock et al. (2011) retrospectively reviewed 59 consecutive LLIF surgeries at their institution. After a thorough review of the medical records, they discovered that 62.7 of the patient's had suffered neurological injury to the lumbar plexus which of course presented as anterior thigh and/or groin pain, burning, numbness, and/or weakness. Luckily, 90% of these patients ostensibly had complete resolution of their symptoms by one year, but 10% did not! In other words, 10% of people still suffered radicular pain (sciatica) even one year after the surgery was over.
In an award-winning paper, which was published in a high impact spine Journal, The Spine Journal, Lykissas et al (2013) followed 451 patients for an average of 16 months following their LLIF surgery. Specifically, these patients were separated into two groups: a rhBMP-2 augmentation group (their LLIF was augmented with rhBMP-2 to help ensure bony fusion) and a non-rhBMP-2 group. Immediately after surgery, approximately 47% of all patients suffered neurological injury. By the last evaluation, 40.3% of the BMP group were still suffering! In the non-BMP group, 27.8% were still suffering symptoms of nerve injury secondary to the procedure. Although, the neurological injuries suffered by the patient's who had their procedure augmented by rhBMP-2 was absolutely ridiculous, so was the group that didn't have their procedure augmented with rhBMP-2.
Although some surgeons have published LLIF results showing much less neurological injury, because of the Lykissas et al results, which was published in one of the major spine journals, I am not recommending that patients/clients choose a surgeon who performs this type of surgery.
They must go back to the drawing board on the LLIF (XLIF) technique!
In fact, they already seem to have a replacement that is in its infancy as we speak. It's called oblique lumbar interbody fusion or OLIF. I will not even comment on this yet because there are hardly any papers on, but there probably will be in another few years. The only thing I will say is that it is an approach to the disc that misses the psoas major muscle and its lumbar plexus and still is retroperitoneal (you don't have to open the peritoneum, which is always a good thing).
This is a very big topic and at this point, I am just not going to cover it. I would be more than happy to discuss the pros and cons of the various types of lumbar arthroplasty via one of my Coaching Sessions.
Recombinant human bone morphogenetic protein-2 (BMP-2) is a powerful biologic (a growth factor) which was designed to help patients establish real bony fusion after their fusion surgery. Typically, non-BMP-2 supplemented lumbar fusion has about 88% chance of actually working (fusing), while fusions augmented with BMP-2 fuse ~ 98% of the time. Who cares if bony fusion occurs? Successful solid bony fusion (which means the two target vertebrae actually fused together into one solid unitk) is highly associated with patient satisfaction rates. In other words, if your fusion is going to work, you better hope for a successful bony fusion. Pseudoarthrosis (failure to fuse) is definitely rated with poor patient satisfaction.
Following early clinical trials, which demonstrated BMP-2 was completely safe, it soon became evident that there were problems associated with its use.
In 2009, a time when BMP-2 was being very heavily used to augment lumbar fusion surgery, Stanford’s Eugene Carragee, M.D., orthopedic spine surgeon (the director of Stanford’s orthopedic surgery department and the editor of “the Spine Journal,”) published a scathing editorial which basically accused the authors of the initial BMP-2 studies of failing to report truthfully on associated BMP-2-related adverse outcomes and complications. He went to great lengths to point out that none of the original investigations reported a single complication. Why? As he pointed out, perhaps it was because some of the authors of the studies were receiving "tens of millions of dollars" directly or indirectly from BMP-2's manufacturer. In this paper, Carragee noted that complications of postoperative cancer development and sterility were obviously overlooked in these original papers even though the FDA was well aware of the trouble. [1] Other complications / adverse effects became apparent which included an out-of-control bone formation phenomenon (epidural ectopic bone formation) and an osteolytic endplate destroying process, both of which continue to be present at this day and age.
Still beating his drum, in 2013 Carragee et al published another BMP-2 paper [2] in the prestigious Journal of Bone and Joint Surgery that further evaluated the risk of new cancer development in patients that received high doses of BMP-2. By pooling the same data from the early studies (all of which is publicly available), Carragee concluded, “A high dose of 40 mg of BMP-2 (as applied during) lumbar spinal arthrodesis (fusion) was associated with an increased risk of new cancer.”
Carragee may have went a little overboard, however, with his drum beating, especially about cancer. In fact, he was later openly criticized by some of his colleagues for going too far by publishing opinions that were not completely supported by the research data. In fact, if memory serves, some of these researchers even called for his resignation from The Spine Journal.
In 2013, Simmonds et al publish the results of a highly anticipated meta-analysis which was paid for by the manufacturer of BMP-2 in order to transparently revisit the cancer and complication issues raised mostly by Carragee. After reviewing the same data that the early researchers reviewed, they concluded that "rhBMP-2 increases rates of fusion" and "evidence of increased cancer incidence is inconclusive." [3]
At approximately the same time, a companion meta-analysis, also funded by the manufacturer, was completed by a very prominent research group in Europe. In their 2013 paper, Fu et al [4] concluded there was "substantial evidence of reporting bias" (talking about the early authors), and that more research was needed before "more reliable estimates of the risk of cancer" following the use of BMP-2 can be put forth. [4]
Therefore, notwithstanding Carragee's claims (based on his own data crunching of those same numbers), a definite link between BMP-2 and cancer development was not established. Much to the chagrin of the trial lawyers I should add.
In 2014, Kelly et al [5] looked at the issue further and published the results of their investigation into whether or not spinal fusion augmented (accompanied) with recombinant human bone morphogenetic protein-2 (BMP-2) increase the incidence (chances of) the development of cancer. Here’s how the study worked: from Medicare records, they pulled out 467,916 patients who had undergone spinal fusion and put them into two groups: the study group (n= 110,808), all of whom had their fusions augmented with BMP-2, and a control group (n=357,108) who did not have BMP-2 augmentation with their fusion. Then, at an average of three years status post fusion, they looked at whether or not these patients developed cancer following their fusion. After crunching the numbers, they concluded that at BMP-2 was not related to the development of cancer. On the contrary, the patients who did not have BMP-2 used during the fusion had a significantly higher rate (P<0.001) of cancer development! [5] [Kelly-2014]
COMMENTS: Even though a link between BMP-2 and cancer has not been firmly established, I agree with the surgeons who do not believe BMP-2 should be used on every single fusion surgery. [5] Just to be safe, let's wait another 5-10 years and see what the research says about BMP-2's safety.
Citations:
1) Carragee et al. A Challenge to Integrity in Spine Publications: Years of Living Dangerously with the Promotion of Bone Growth Factors. Spine J 2011;11:463-468.
2) Carragee et al. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am 2013;95:1537-1545.
3) Simmonds et al. Safety and Effectiveness of Recombinant Human Bone Morphogenetic Protein-2 for Spinal Fusion. A meta-analysis of patient-participant data. Ann Intern Med 2013;158:877-889.
4) Fu et al. Effectiveness and Harms of Recombinant Human Bone Morphogenetic Protein-2 in Spinal Fusion: a Systematic Review and Meta-Analysis. Ann Intern Med 2013;158:890-902.
5) Kelly et al. Cancer Risk from Bone Morphogenetic Protein Exposure in Spinal Arthrodesis. J Bone Joint Surg Am 2014;96:1417-1422.
6) Riew and Carragee. Commentary: Despite Reports of Catastrophic Complications, Why Recombinant Human Bone Morphogenetic Protein-2 Should Be Available for Use In Anterior Cervical Spine Surgery. Spine J 2012; 12:894-899.
Since fusion doesn't have the greatest track record, and its failure has the potential to result in horrible life-long pain, please consider trying all other types of non-fusion care first, which include bedrest; permanent activity modifications; medication; bracing; physical therapy & exercise; low-force chiropractic care; acupuncture; injective procedures; and (if stenosis or recurrent herniation are the problems) a decompressive spine surgery, such as laminotomy, laminectomy and/or foraminotomy.
Although I won't get into all the common types of conservative care, I will talk about medication, decompressive surgery, epidural steroid injections, and experimental treatments him.
 Hands down, the most common type of treatment for low back pain is the use of medication, which is prescribed for 80% of patients entering their primary care physician's office on the first visit. [23]
Hands down, the most common type of treatment for low back pain is the use of medication, which is prescribed for 80% of patients entering their primary care physician's office on the first visit. [23]
Over-the-counter medications, such as acetaminophen (Tylenol) and ibuprofen (Advil) are also effective for certain types of pain.
The most common of prescription medications him him and are nonsteroidal anti-inflammatories, (NSAIDs) muscle relaxers, and opioids. [23-25] However, benzodiazepine, corticosteroids, antidepressants, and antiepileptic medication are also used, only less frequently. [26]
Surprisingly, there is not much quality research published on the long-term efficacy (> 6 months) use of any medication for the treatment of chronic back pain, for the Lions' share of data comes from studies with only four months or less of treatment.
One very high quality meta-analysis (level I evidence) recently looked at the efficacy of opioids (Vicodin, Percocet, or MS Contin) for the treatment of chronic low back pain. [21] Specifically, after pooling the data from 5,540 patient who had participated in only quality randomized controlled trials with placebo, Chaparro et al. reported that in the short term (four months) opioids were more effective than a placebo (a fake sugar pill) at reducing chronic back pain and increasing patient function; furthermore, there were no serious adverse effects reported with the opioids. [21] This team of researchers also compared NSAIDs to opioids in failed to present strong or even moderate evidence that NSAIDs were as effective as opioids. In other words, opioids are still better for the treatment of chronic low back pain. [21]
Chou et al. [22] published the results of their systematic review which pooled data from randomized control trials that investigated different types of medication for the treatment of chronic back pain. They concluded that there was "good" evidence supporting a four-month trial of NSAIDs, muscle relaxers, or tricyclic antidepressants for the treatment of chronic back pain. [22] On the other hand, the also discovered that corticosteroids were ineffective for the treatment of chronic low back pain with or without sciatica. [22]
Because every patient has unique biochemistry, what works for one patient may not work for another; therefore, I believe that all of the aforementioned medications must be tried in attempts to prevent fusion. Such experimentation, however, must be carried out under the direct supervision of your primary care physician and or chronic pain specialist.
Anecdotally, chronic neuropathic pain in the lower extremity seems to respond favorably to either Neurontin or, it's a more modern form, Lyrica. Unfortunately, both medications (Neurontin >Lyrica) have the unwanted side effect of "brain fog" which takes some getting used to; I could never get used to it, and had to discontinue both medications. Him him
For patients with very large or recurrent disc herniation, as well as stenosis, a choice between decompressive surgery (i.e., laminectomy, laminotomy, foraminotomy) and interbody fusion may be offered.
If this happens, which one do you try? And, if the decompression is tried, what happens if it fails? I mean, will it decrease the chances of having a successful subsequent fusion? Although the answer to the first question will depend on many factors, so I cannot answer it here. [Remember I do offer a Coaching Service where I can help you make this decision] The answer to the last question is probably not. How do I know? I actually designed and published a medical investigation geared at answering this very question. [20]
Specifically, in 2014, Gillard et al. [Dr. Gillard, that's me] [20] published the results of a rare study that compared the fusion outcomes of two groups: a group that underwent decompression prior to fusion, and a group that did not. At an average follow-up of 40.4 months, both groups demonstrated high patient satisfaction; however, there was no statistical difference between groups with regard to clinical outcomes at the last follow-up. [20] In other words, at least in this small study, trying a decompressive surgery prior to fusion does not seem to negatively affect medium term clinical outcomes.
There are other "experimental" procedures out there, many of which I have discussed on my Spine Research Page, which have been touted as fusion alternatives. These include, SED, discTRODE, Nucleoplasty, PIRFT, RF-Denervation, Spinal Cord Stimulation, IDET, Chemonucleolysis, Prolotherapy, Intradiscal PRP Injection, and Intradiscal Steroid Injection. Unfortunately, the patent holders of these procedures still have not put them through vigorous randomized controlled trials; therefore, I just don't believe that they should be considered at this time. [19] I have written about many of these procedures on my research page, so if you want to know more, that's the place to go.
Intradiscal biacuplasty, however, is one fusion alternative that may have demonstrated its efficacy (safe and effective treatment) and may be worth considering. I have already discussed this technique in depth on the disc herniation page, so if you're interested, please click here to learn more.
Although epidural steroid injections are typically thought of as a treatment for lower extremity pain, they may also help, at least temporarily, with discogenic low back pain and are certainly worth a try. Again, I have already discussed this technique in depth on the disc herniation page, so please click here if you want to learn more.
Anecdotally, it is well known discogenic pain syndrome (i.e., pain coming from an annular tear, endplate pain syndrome, or disc herniation) does refer pain into the sacroiliac (SI) joints in some patients.
Therefore, before undergoing fusion for chronic discogenic pain, SI joint blocks should be performed in order to rule the SI joint out as a pain generator. You certainly would not want to have lumbar interbody fusion if the pain generator was really the SI joint! If this were the case, that fusion would have been a complete waste of time can cause serious harm to your lumbar spine.
The SI joints also may become a new source of low back pain after a successful lumbar fusion. [29] This phenomenon occurs because, as mentioned before, fusion screws up the biomechanics of the lumbar spine and not only overloads the segment above the fusion, but also overloads both sacroiliac joints, which can lead to premature degeneration and pain. In fact, in one 2008 study, Ha et al. [29] demonstrated that 75% of fusion patients had CT evidence of significant SI degeneration five years after their fusion, as compared to 38% of asymptomatic people in a control group who also had the CT performed at five years. [29]
A dysfunctional and painful SI joint, which may occur in up to 18% of people with chronic low back pain, [27] is very hard, if not impossible, to clinically diagnose. [28] Computed tomographic findings of joint destruction continues to be the gold standard. [30]
1) Nachemson AF, Schultz AB, Berkson MH. Mechanical properties of human lumbar spine motion segments; influences of age, sex, distal level, and degeneration. Spine 1997;4(1):1-8.
2) Drum D. the vertebral motor unit and intervertebral foramen. In: The research stat of spinal manipulation therapy, M. Goldstein, Ed. US Department health education and welfare, HIH Bethesda, MD (1975).
3) Djurasovic M, Glassman SD, Dimar II Jr, et al. Does fusions status correlate with patient outcomes in lumbar spinal fusion? Spine 2011;36:404-409.
4) Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery: the case for restraint. N Engl J Med 2004; 350:722-726.
5) Vamvanij V, Fredrickson BE, Thorpe JM, et al. Surgical treatment of intervertebral this disruption. J Spinal Discord 1998; 11:375-382. *
6) Bono CM, Lee CK. The influence of subdiagnosis on radiographic and clinical outcomes after lumbar fusion for Degenerative Disc Disorders: an analysis of literature from two decades. spine 2005; 30 (2): 227-234.
7) Harms JG, Jeszenszky D. Die posteriore, lumbale, interkorporelle Fusion in unilateraler transforaminaler Technik. Orthop Traumatol 1998; 10:90-102.
8) Fraser RD: Interbody, posterior, and combined lumbar fusion. Spine 1995; 20 (24 Suppl):167S-177S.
9) Stonecipher T, Wright S: Posterior lumbar interbody fusion with facet-screw fixation. Spine 1989; 14:468-471.
10) Yajun W, Yue Z, Xiuxin H, Cui C. A meta-analysis of artificial total disc replacement versus fusion for lumbar degenerative disc disease. Eur Spine J 2010; 19:1250-1261.
11) Glassman SD, Carreon LY, Djurasovic M, et al. "Lumbar Fusion Outcomes Stratified by Specific Diagnostic Indication: 2008 Outstanding Paper Award Runner up" Spine J 2009;9: 13-21.
12) Jorgenson SS, Lowe TG, France J, Sabin J. "A Prospective Analysis of Autograft Versus Allograft and Posterolateral Lumbar Fusion in the Same Patient. Spine 1994; 19 (18): 2048-2053.
13) Glassman SD, Howard JM, Sweet A, Carreon LY. "Complications and concerns with osteobiologics for spine fusion in clinical practice. Spine 2010; 35:1621-1628.
14) Greiner-Perth R, Boehm H, Allam Y, et al. Reoperation rate after instrumented posterior lumbar interbody fusion: a report on 1,680 cases. spine 2004;29:2516-2520.
15) Ren C, Song Y, Liu L, Xue Y. Adjacent segment degeneration and disease after lumbar fusion compared with motion-preserving procedures. a meta-analysis. Eur J Orthop Surg Traumatol 2014; ahead of print. PMID: 24728779.
16) Harrop JS, Youssef JA, Maltenfort M, et al. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine 2008; 33:1701-1707.
17) Celestre PC, Montgomery SR, Kupperman AI, et al. Lumbar clinical adjacent segement pathology: predilection for proximal levels. Spine 2014; 39:172-176.
18) Akamaru T, Kawahara N, Yoon T, et al. Adjacent motion segment after a simulated lumbar fusion in different sagittal alignments: a biomechanical analysis. Spine 2003;28:1560-1566.
19) Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine 2009;34:1078-1093.
20) Gillard DM, Corenman DS, Dornan GJ. Failed less invasive lumbar spine surgery as a predictor of subsequent fusion outcomes. Int Orthop 2014;38:811-815.
21) Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared with placebo or other treatments for chronic low back pain. spine 2014; 39:556-563.
22) Chou R, Huffman LH. Medication for acute and chronic low back pain: a review of the evidence from an American pain Society/American College of physicians clinical practice guidelines. Ann Intern Med 2007; 147:505-514.
23) Cherkin DC, et al. Medication use for low back pain in primary care. Spine 1998; 23:607-614.
24) Bernstein E, et al. The use of muscle relaxant medication in acute low back pain. Spine 2004; 29:1346-1351.
25) Luo X, et al. Patterns and trends in opioid use among individuals with low back pain in the United States. Spine 2004; 29:884-890; discussion 891.
26) Di ID, et al. A survey of primary care physician practice patterns and adherence to acute low back pain problem guidelines. Arch Fam Med 2000;9: 1015-1021.
27) Fortin JD, Aprill CN, Ponthieux B, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part II: clinical evaluation. Spine 1994; 13:1483-1489.
28) Dreyfuss P, Michaelsen M, Pauza K, et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine 1996; 21:2594-2602.
29) Ha KY, Lee JS, Kim KW. Degeneration of sacroiliac joint after instrumented lumbar or lumbosacral fusion: a prospective cohort study over five-year follow-up. Spine 2008;33:1192-1198.
30) Borlaza GS, et al. Computed tomography in the evaluation of sacroiliac arthritis. Radiology 1981;139:437-440.
31) Albee FH: Transplantation of a portion of the tibia into the spine for Pott’s disease. JAMA 57:885-86, 1911.
32) Wiltse L. Proceedings: lumbar spine: posterolateral fusion. J Bone Joint Surg Br. 1975; 57:261.
33) Abe NE, et al. Lumbar intradiscal pressure after posterolateral fusion and pedicle screw fixation. Tohoku J. Exp. Med., 1998; 186:243-253.
34) Thomsen K, christensen FB, Eiskjaer SP, et al. 1997 Volvo award winner in clinical studies: the effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: a prospective, randomized clinical study. Spine 1997; 22:2813-2822.
35) Djurasovic M, Glassman SD, Dimar II JR, et al. Does fusion status correlate with patient outcomes and lumbar spinal fusion. Spine 2011; 36:404-409.
36) Christensen FB, Hansen ES, Eiskjaer SP, et al. Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion with titanium Cotrel-Dubousset instrumentation. A prospective, randomized clinical study of 146 patients. Spine 2002; 27:2674-2683.
37) Videbaek TS, Christensen FB, Soegaard R, et al. Circumferential fusion improves outcome in comparison with instrumented posterolateral fusion: long-term results of a randomized clinical trial. Spine 2006; 31: 2875-2880.
38) Mardjetko SM, Connolly PJ, Shott S. Degenerative lumbar spondylolisthesis: a meta-analysis of the literature 1970-1993. Spine 1994;19:2256S-2265S.
39) Rajaee SS, Bae HW, Kanim LEA, et al. Spinal fusion in the United States. Spine 2012;37:67-76.
40) Bydon M, et al. Lumbar fusion vs. non-operative management for treatment of discogenic low backk pain: a systematic review and meta-analysis of randomized controlled trials. J Spinal Discord Tech 2014; PMID 24346052 (ahead of print).
41) Saltychev M, et al. Lumbar fusion compared with conservative treatment in patients with chronic low back pain: a meta-analysis. Int J Rehabil Res. 2014; 37:2-8.
42) Umeta RS, Avanzi O. Techniques of lumbar-sacral spine fusion in spondylosis: systematic literature review and meta-analysis of randomized clinical trials. Spine J 2011;11:668-676.
43) Carragee EJ, Chu G, Rohatqi R, et al. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg AM 2013; 95:1537-1545.
44) Cloward RB. The treatment of ruptured lumbar intervertebral disc by vertebral body fusion III. Method of use of banked bone. Ann Surg 1952; 136:987-992.
45) Cloward RB. The treatment of ruptured lumbar intervertebral discs by the vertebral body fusion I. indications, operative technique, aftercare. J Neurosurg 1953; 10: 154-168.
46) Farrokhi MR, et al. Posterolateral versus posterior interbody fusion in isthmic spondylolisthesis. J Neurotrama 2012; 29:1567-1573.
47) Barnes B, Rodts Jr. GE, Haid Jr. RW, et al. Allograft implants for posterior lumbar interbody fusion: results comparing cylindrical dowels and impacted wedges. Neurosurgery 2002; 51:1191-1198; discussion 1198.
48) Fritzell P, Hagg O, Wessberg P et al. 2001 Volvo award winner in clinical studies: lumbar fusion versus nonsurgical treatment for chronic low back pain. A multicenter randomized control trial from Swedish lumbar spine study group. Spine 2001; 26:2521-2534.
49) Humphreys SC, Hodges SC, Patwardhan AG, et al. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine 2001; 26:567-571.
50) Hee HT, Castro FP, Majd ME, et al. Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion: analysis of complications and predictive factors. J Spinal Disord 2001;14:523-540.
51) Hackenberg L, Halm H, Bullmann V, et al. Transforaminal lumbar interbody fusion : a safe technique with satisfactory 3 to 5 year results. Eur Spine J 2005; 14:551-558.
52) Cooper GS, Kou TD. Risk of cancer following lumbar fusion surgery with recombinant human bone morphogenetic protein-2 (rH-BMP-2). Spine 2013; 38:1862-1868.
53) Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared to nonoperative treatment for degenerative spondylolisthesis. Four-year results in the spine patient outcome research trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 2009; 91:1295-1304.
54) Penta M, Fraser RD. Anterior lumbar interbody fusion; a minimum 10-year follow-up. Spine 1997; 22:2429-2434.
55) Lane JD, Moore ES. Transperitoneal approach to the intervertebral disc in the lumbar area. Annals Surg 1948; 127:537-551.
56) Flynn JC, Price CT. Sexual complications of anterior fusion of the lumbar spine. Spine 1984;9:489-492.
57) Tiusanen H, et al. Retrograde ejaculation after anterior lumbar interbody fusion. Eur Spine J 1995; 4:339-342.
58) Sasso RC, Burkus JK, LeHuec JC. Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine 2003;10:1023-1026.